Intramuscularly administered neuraminidase inhibitor peramivir is effective against lethal H5N1 influenza virus in mice
- PMID: 18573280
- PMCID: PMC2847517
- DOI: 10.1016/j.antiviral.2008.05.012
Intramuscularly administered neuraminidase inhibitor peramivir is effective against lethal H5N1 influenza virus in mice
Abstract
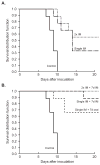
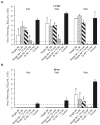
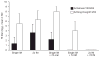
The replication efficiency and multi-organ dissemination of some influenza A (H5N1) viruses requires a rapid re-evaluation of the available antiviral strategies. We assessed five regimens of the neuraminidase (NA) inhibitor peramivir in mice inoculated with H5N1 virus. The regimens differed by: (1) frequency of administration on first day (once vs twice); (2) duration of administration (1 day vs 8 days); (3) route of administration (intramuscular [IM] injection alone or followed by oral administration). In all regimens, BALB/c mice were administered 30 mg/kg peramivir IM 1 h after lethal challenge with 5 MLD(50) of A/Vietnam/1203/04 (H5N1) influenza virus. When given only on the day of inoculation, a single IM injection produced a 33% survival rate, which increased to 55% with two injections. Eight-day regimens significantly increased survival; two IM injections followed by seven daily IM injections was the most effective regimen (100% survival; inhibition of replication in lungs and brain). When this 8-day regimen began at 24h after inoculation, 78% of mice survived; 56% survived when treatment began at 48 hours. Anti-HA antibody titer differed with the peramivir regimen and corresponded to the severity of disease. Overall, our results demonstrate that IM administration of peramivir is effective in promoting the survival of mice infected with systemically replicating H5N1 virus.
Figures

Similar articles
-
Therapeutic efficacy of peramivir against H5N1 highly pathogenic avian influenza viruses harboring the neuraminidase H275Y mutation.Antiviral Res. 2017 Mar;139:41-48. doi: 10.1016/j.antiviral.2016.12.011. Epub 2016 Dec 22. Antiviral Res. 2017. PMID: 28012921
-
Therapeutic activity of intramuscular peramivir in mice infected with a recombinant influenza A/WSN/33 (H1N1) virus containing the H275Y neuraminidase mutation.Antimicrob Agents Chemother. 2012 Aug;56(8):4375-80. doi: 10.1128/AAC.00753-12. Epub 2012 Jun 4. Antimicrob Agents Chemother. 2012. PMID: 22664977 Free PMC article.
-
Prophylactic activity of intramuscular peramivir in mice infected with a recombinant influenza A/WSN/33 (H1N1) virus containing the H274Y neuraminidase mutation.Antimicrob Agents Chemother. 2010 Jul;54(7):2819-22. doi: 10.1128/AAC.01681-09. Epub 2010 Apr 19. Antimicrob Agents Chemother. 2010. PMID: 20404128 Free PMC article.
-
Peramivir (Rapivab): an IV neuraminidase inhibitor for treatment of influenza.Med Lett Drugs Ther. 2015 Feb 2;57(1461):17-9. Med Lett Drugs Ther. 2015. PMID: 25629811 Review. No abstract available.
-
Laninamivir octanoate: a new long-acting neuraminidase inhibitor for the treatment of influenza.Expert Rev Anti Infect Ther. 2011 Oct;9(10):851-7. doi: 10.1586/eri.11.112. Expert Rev Anti Infect Ther. 2011. PMID: 21973296 Review.
Cited by
-
Clinical Effectiveness of Intravenous Peramivir Compared With Oseltamivir in Patients With Severe Influenza A With Primary Viral Pneumonia: A Randomized Controlled Study.Open Forum Infect Dis. 2020 Nov 18;8(1):ofaa562. doi: 10.1093/ofid/ofaa562. eCollection 2021 Jan. Open Forum Infect Dis. 2020. PMID: 33447633 Free PMC article.
-
Combinations of oseltamivir and peramivir for the treatment of influenza A (H1N1) virus infections in cell culture and in mice.Antiviral Res. 2010 Oct;88(1):38-44. doi: 10.1016/j.antiviral.2010.07.003. Epub 2010 Jul 13. Antiviral Res. 2010. PMID: 20633577 Free PMC article.
-
Peramivir: A Novel Intravenous Neuraminidase Inhibitor for Treatment of Acute Influenza Infections.Front Microbiol. 2016 Mar 31;7:450. doi: 10.3389/fmicb.2016.00450. eCollection 2016. Front Microbiol. 2016. PMID: 27065996 Free PMC article. Review.
-
Peramivir: A Review in Uncomplicated Influenza.Drugs. 2018 Sep;78(13):1363-1370. doi: 10.1007/s40265-018-0981-8. Drugs. 2018. PMID: 30196350 Review.
-
Animal Models for Influenza Virus Pathogenesis and Transmission.Viruses. 2010;2(8):1530-1563. doi: 10.3390/v20801530. Viruses. 2010. PMID: 21442033 Free PMC article.
References
-
- Babu YS, Chand P, Bantia S, Kotian PL, Dehghani A, El-Kattan Y, Lin TH, Hutchison TL, Elliot AJ, Parker CD, Ananth SL, Horn LL, Laver GW, Montgomery JA. BCX-1812 (RWJ-270201): discovery of a novel, highly potent, orally active and selective influenza neuraminidase inhibitor through structure-based drug design. J Med Chem. 2000;43:3482–3486. - PubMed
-
- Bantia S, Parker CD, Ananth SL, Horn LL, Andries K, Chand P, Kotian PL, Dehghani A, El-Kattan Y, Lin T, Hutchison TL, Montgomery JA, Kellog DL, Babu YS. Comparison of the anti-influenza virus activity of RWJ-270201 with those of oseltamivir and zanamivir. Antimicrob Agents Chemother. 2001;45:1162–1167. - PMC - PubMed
-
- Bantia S, Arnold CS, Parker CD, Upshaw R, Chand P. Anti-influenza virus activity of peramivir in mice with single intramuscular injection. Antiviral Res. 2006;69:39–45. - PubMed
-
- Barroso L, Treanor J, Gubareva L, Hayden FG. Efficacy and tolerability of the oral neuraminidase inhibitor peramivir in experimental human influenza: randomized, controlled trials for prophylaxis and treatment. Antivir Ther. 2005;10:901–910. - PubMed
-
- Baum EZ, Wagaman PC, Ly I, Turchi I, Le J, Bucher D, Bush K. A point mutation in influenza B neuraminidase confers resistance to peramivir and loss of slow binding. Antiviral Res. 2003;59:13–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

