Localized cell and drug delivery for auditory prostheses
- PMID: 18573323
- PMCID: PMC3073348
- DOI: 10.1016/j.heares.2008.06.003
Localized cell and drug delivery for auditory prostheses
Abstract
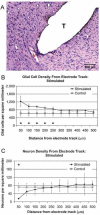
Localized cell and drug delivery to the cochlea and central auditory pathway can improve the safety and performance of implanted auditory prostheses (APs). While generally successful, these devices have a number of limitations and adverse effects including limited tonal and dynamic ranges, channel interactions, unwanted stimulation of non-auditory nerves, immune rejection, and infections including meningitis. Many of these limitations are associated with the tissue reactions to implanted auditory prosthetic devices and the gradual degeneration of the auditory system following deafness. Strategies to reduce the insertion trauma, degeneration of target neurons, fibrous and bony tissue encapsulation, and immune activation can improve the viability of tissue required for AP function as well as improve the resolution of stimulation for reduced channel interaction and improved place-pitch and level discrimination. Many pharmaceutical compounds have been identified that promote the viability of auditory tissue and prevent inflammation and infection. Cell delivery and gene therapy have provided promising results for treating hearing loss and reversing degeneration. Currently, many clinical and experimental methods can produce extremely localized and sustained drug delivery to address AP limitations. These methods provide better control over drug concentrations while eliminating the adverse effects of systemic delivery. Many of these drug delivery techniques can be integrated into modern auditory prosthetic devices to optimize the tissue response to the implanted device and reduce the risk of infection or rejection. Together, these methods and pharmaceutical agents can be used to optimize the tissue-device interface for improved AP safety and effectiveness.
Figures


Similar articles
-
Design and fabrication of multichannel cochlear implants for animal research.J Neurosci Methods. 2007 Oct 15;166(1):1-12. doi: 10.1016/j.jneumeth.2007.05.013. Epub 2007 May 21. J Neurosci Methods. 2007. PMID: 17727956 Free PMC article.
-
Factors influencing neurotrophic effects of electrical stimulation in the deafened developing auditory system.Hear Res. 2008 Aug;242(1-2):86-99. doi: 10.1016/j.heares.2008.06.002. Epub 2008 Jun 7. Hear Res. 2008. PMID: 18573324 Free PMC article.
-
Chronic depolarization enhances the trophic effects of brain-derived neurotrophic factor in rescuing auditory neurons following a sensorineural hearing loss.J Comp Neurol. 2005 May 30;486(2):145-58. doi: 10.1002/cne.20564. J Comp Neurol. 2005. PMID: 15844207 Free PMC article.
-
Neurotrophic factors and neural prostheses: potential clinical applications based upon findings in the auditory system.IEEE Trans Biomed Eng. 2007 Jun;54(6 Pt 1):1138-48. doi: 10.1109/TBME.2007.895375. IEEE Trans Biomed Eng. 2007. PMID: 17551571 Free PMC article. Review.
-
Optogenetic stimulation of the auditory pathway for research and future prosthetics.Curr Opin Neurobiol. 2015 Oct;34:29-36. doi: 10.1016/j.conb.2015.01.004. Epub 2015 Jan 28. Curr Opin Neurobiol. 2015. PMID: 25637880 Review.
Cited by
-
Electrochemically Enhanced Delivery of Pemetrexed from Electroactive Hydrogels.Polymers (Basel). 2022 Nov 16;14(22):4953. doi: 10.3390/polym14224953. Polymers (Basel). 2022. PMID: 36433079 Free PMC article.
-
AAV-Mediated Neurotrophin Gene Therapy Promotes Improved Survival of Cochlear Spiral Ganglion Neurons in Neonatally Deafened Cats: Comparison of AAV2-hBDNF and AAV5-hGDNF.J Assoc Res Otolaryngol. 2019 Aug;20(4):341-361. doi: 10.1007/s10162-019-00723-5. Epub 2019 Jun 20. J Assoc Res Otolaryngol. 2019. PMID: 31222416 Free PMC article.
-
Future approaches for inner ear protection and repair.J Commun Disord. 2010 Jul-Aug;43(4):295-310. doi: 10.1016/j.jcomdis.2010.04.001. Epub 2010 Apr 8. J Commun Disord. 2010. PMID: 20430401 Free PMC article. Review.
-
Targeted delivery of brain-derived neurotrophic factor for the treatment of blindness and deafness.Int J Nanomedicine. 2015 Apr 30;10:3245-67. doi: 10.2147/IJN.S77480. eCollection 2015. Int J Nanomedicine. 2015. PMID: 25995632 Free PMC article. Review.
-
Virally Mediated Overexpression of Glial-Derived Neurotrophic Factor Elicits Age- and Dose-Dependent Neuronal Toxicity and Hearing Loss.Hum Gene Ther. 2019 Jan;30(1):88-105. doi: 10.1089/hum.2018.028. Epub 2018 Sep 5. Hum Gene Ther. 2019. PMID: 30183384 Free PMC article.
References
-
- Altar CA, Boylan CB, Fritsche M, Jones BE, Jackson C, Wiegand SJ, Lindsay RM, Hyman C. Efficacy of brain-derived neurotrophic factor and neurotrophin-3 on neurochemical and behavioral deficits associated with partial nigrostriatal dopamine lesions. J Neurochem. 1994;63:1021–32. - PubMed
-
- Arnold A, Ehrenberger K, Frenz M, Pratisto H, Weber HP, Altermatt HJ, Felix D. Experimental erbium laser surgery in the guinea pig cochlea: its use in the study of afferent cochlear neurotransmitters. Eur Arch Otorhinolaryngol. 1996;253:460–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

