Immunological link between primary graft dysfunction and chronic lung allograft rejection
- PMID: 18573422
- PMCID: PMC2790810
- DOI: 10.1016/j.athoracsur.2008.03.073
Immunological link between primary graft dysfunction and chronic lung allograft rejection
Abstract
Background: Primary graft dysfunction (PGD) in the immediate post-lung transplant period strongly increases the risk of chronic rejection (broncholitis obliterans syndrome). Here, we hypothesized that PGD-induced inflammation augments alloimmunity, thereby predisposing to broncholitis obliterans syndrome.
Methods: Primary graft dysfunction and broncholitis obliterans syndrome were diagnosed according to the established International Society for Heart and Lung Transplantation criteria. Anti-human leukocyte antigen (HLA) alloantibodies were analyzed using Flow-PRA. Donor HLA class II-specific T cells were analyzed using interferon (IFN)-gamma ELISPOT. Serum levels of 25 cytokines and chemokines were measured using LUMINEX.
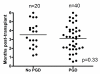
Results: Of the 127 subjects, 29 (22.8%) had no PGD (grade 0), 42 (33.2%) had PGD-1, 36 (28.3%) had PGD-2, and 20 (15.7%) had PGD-3. Patients with PGD grades 1 to 3 (PGD(1-3)) had elevated proinflammatory mediators MCP-1, IP-10, interleukin (IL)-1 beta, IL-2, IFN-gamma, and IL-12 in the sera during the early posttransplant period compared with patients with PGD grade 0 (PGD(0)). On serial analysis, PGD(1-3) patients revealed increased development of de novo anti-HLA-II (5 years: 52.2% versus PGD(0) 13.5%, p = 0.008). However, no difference was found in anti-HLA-I alloantibody development (PGD(1-3) patients 48% versus PGD(0) 39.6%, p = 0.6). Furthermore, PGD(1-3) patients had increased frequency of donor HLA class II-specific CD4(+) T cells [(91.4 +/- 19.37) x 10(-6) versus (23.6 +/- 15.93) x 10(-6), p = 0.003].
Conclusions: Primary graft dysfunction induces proinflammatory cytokines that can upregulate donor HLA-II antigens on the allograft. Increased donor HLA-II expression along with PGD-induced allograft inflammation promotes the development of donor specific alloimmunity. This provides an important mechanistic link between early posttransplant lung allograft injury and reported association with broncholitis obliterans syndrome.
Figures



References
-
- Sundaresan S, Trulock EP, Mohanakumar T, Cooper JD, Patterson GA. Prevalence and outcome of bronchiolitis obliterans syndrome after lung transplantation. Washington University Lung Transplant Group. Ann Thorac Surg. 1995;60(5):1341–1346. discussion 1346-1347. - PubMed
-
- Sundaresan S, Mohanakumar T, Smith MA, et al. HLA-A locus mismatches and development of antibodies to HLA after lung transplantation correlate with the development of bronchiolitis obliterans syndrome. Transplantation. 1998;65(5):648–653. - PubMed
-
- Jaramillo A, Smith MA, Phelan D, et al. Development of ELISA-detected anti-HLA antibodies precedes the development of bronchiolitis obliterans syndrome and correlates with progressive decline in pulmonary function after lung transplantation. Transplantation. 1999;67(8):1155–1161. - PubMed
-
- Jaramillo A, Zhang L, Mohanakumar T. Binding of anti-HLA class I antibodies to airway epithelial cells induces activation and growth factor production and indirectly upregulates lung fibroblast proliferation. J Heart Lung Transplant. 2001;20(2):166. - PubMed
-
- Bharat A, Narayanan K, Street T, et al. Early posttransplant inflammation promotes the development of alloimmunity and chronic human lung allograft rejection. Transplantation. 2007;83(2):150–158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

