Two subunits specific to the PBAP chromatin remodeling complex have distinct and redundant functions during drosophila development
- PMID: 18573871
- PMCID: PMC2519717
- DOI: 10.1128/MCB.00747-08
Two subunits specific to the PBAP chromatin remodeling complex have distinct and redundant functions during drosophila development
Abstract
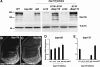
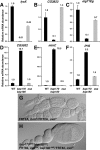
Chromatin remodeling complexes control the availability of DNA binding sites to transcriptional regulators. Two distinct conserved forms of the SWI/SNF class of complexes are characterized by the presence of specific accessory subunits. In Drosophila, the core Brahma complex associates either with Osa to form the BAP complex or with Bap170 and Bap180 to form the PBAP complex. osa mutations reproduce only a subset of the developmental phenotypes caused by mutations in subunits of the core complex. To test whether the PBAP complex performs the remaining functions, we generated mutations in bap170 and bap180. Surprisingly, we found that Bap180 is not essential for viability, although it is required in ovarian follicle cells for normal eggshell development. Bap170 is necessary to stabilize the Bap180 protein, but a mutant form that retains this function is sufficient for both survival and fertility. The two subunits act redundantly to allow metamorphosis; using gene expression profiling of bap170 bap180 double mutants, we found that the PBAP complex regulates genes involved in tissue remodeling and immune system function. Finally, we generated mutants lacking Bap170, Bap180, and Osa in the germ line to demonstrate that the core Brahma complex can function in oogenesis without any of these accessory subunits.
Figures





Similar articles
-
Bap170, a subunit of the Drosophila PBAP chromatin remodeling complex, negatively regulates the EGFR signaling.Genetics. 2010 Sep;186(1):167-81. doi: 10.1534/genetics.110.118695. Epub 2010 Jun 15. Genetics. 2010. PMID: 20551433 Free PMC article.
-
Differential targeting of two distinct SWI/SNF-related Drosophila chromatin-remodeling complexes.Mol Cell Biol. 2004 Apr;24(8):3077-88. doi: 10.1128/MCB.24.8.3077-3088.2004. Mol Cell Biol. 2004. PMID: 15060132 Free PMC article.
-
Functional differentiation of SWI/SNF remodelers in transcription and cell cycle control.Mol Cell Biol. 2007 Jan;27(2):651-61. doi: 10.1128/MCB.01257-06. Epub 2006 Nov 13. Mol Cell Biol. 2007. PMID: 17101803 Free PMC article.
-
Composition and functional specificity of SWI2/SNF2 class chromatin remodeling complexes.Biochim Biophys Acta. 2005 Jan 11;1681(2-3):59-73. doi: 10.1016/j.bbaexp.2004.10.005. Epub 2004 Nov 23. Biochim Biophys Acta. 2005. PMID: 15627498 Review.
-
Deciphering Subunit-Specific Functions within SWI/SNF Complexes.Cell Rep. 2017 Feb 28;18(9):2075-2076. doi: 10.1016/j.celrep.2017.02.045. Cell Rep. 2017. PMID: 28249153 Review.
Cited by
-
Chromatin-Remodelling ATPases ISWI and BRM Are Essential for Reproduction in the Destructive Pest Tuta absoluta.Int J Mol Sci. 2022 Mar 17;23(6):3267. doi: 10.3390/ijms23063267. Int J Mol Sci. 2022. PMID: 35328688 Free PMC article.
-
Understanding the words of chromatin regulation.Cell. 2009 Jan 23;136(2):200-6. doi: 10.1016/j.cell.2009.01.009. Cell. 2009. PMID: 19167321 Free PMC article.
-
ATP-dependent chromatin remodeling in T cells.Biochem Cell Biol. 2012 Feb;90(1):1-13. doi: 10.1139/o11-042. Epub 2011 Oct 14. Biochem Cell Biol. 2012. PMID: 21999456 Free PMC article. Review.
-
Bap170, a subunit of the Drosophila PBAP chromatin remodeling complex, negatively regulates the EGFR signaling.Genetics. 2010 Sep;186(1):167-81. doi: 10.1534/genetics.110.118695. Epub 2010 Jun 15. Genetics. 2010. PMID: 20551433 Free PMC article.
-
A genome-wide gene function prediction resource for Drosophila melanogaster.PLoS One. 2010 Aug 12;5(8):e12139. doi: 10.1371/journal.pone.0012139. PLoS One. 2010. PMID: 20711346 Free PMC article.
References
-
- Andres, A. J., and C. S. Thummel. 1992. Hormones, puffs and flies: the molecular control of metamorphosis by ecdysone. Trends Genet. 8132-138. - PubMed
-
- Bandura, J. L., E. L. Beall, M. Bell, H. R. Silver, M. R. Botchan, and B. R. Calvi. 2005. humpty dumpty is required for developmental DNA amplification and cell proliferation in Drosophila. Curr. Biol. 15755-759. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
