Increased insulin action in SKIP heterozygous knockout mice
- PMID: 18573875
- PMCID: PMC2519744
- DOI: 10.1128/MCB.01990-06
Increased insulin action in SKIP heterozygous knockout mice
Abstract
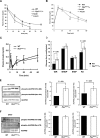
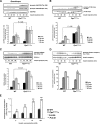
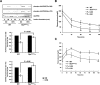
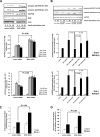
Insulin controls glucose homeostasis and lipid metabolism, and insulin impairment plays a critical role in the pathogenesis of diabetes mellitus. Human skeletal muscle and kidney enriched inositol polyphosphate phosphatase (SKIP) is a member of the phosphatidylinositol 3,4,5-trisphosphate phosphatase family (T. Ijuin et al. J. Biol. Chem. 275:10870-10875, 2000; T. Ijuin and T. Takenawa, Mol. Cell. Biol. 23:1209-1220, 2003). Previous studies showed that SKIP negatively regulates insulin-induced phosphatidylinositol 3-kinase signaling (Ijuin and Takenawa, Mol. Cell. Biol. 23:1209-1220, 2003). We now have generated mice with a targeted mutation of the mouse ortholog of the human SKIP gene, Pps. Adult heterozygous Pps mutant mice show increased insulin sensitivity and reduced diet-induced obesity with increased Akt/protein kinase B (PKB) phosphorylation in skeletal muscle but not in adipose tissue. The insulin-induced uptake of 2-deoxyglucose into the isolated soleus muscle was significantly enhanced in Pps mutant mice. A hyperinsulinemic-euglycemic clamp study also revealed a significant increase in the rate of systemic glucose disposal in Pps mutant mice without any abnormalities in hepatic glucose production. Furthermore, in vitro knockdown studies in L6 myoblast cells revealed that reduction of SKIP expression level increased insulin-stimulated Akt/PKB phosphorylation and 2-deoxyglucose uptake. These results imply that SKIP regulates insulin signaling in skeletal muscle. Thus, SKIP may be a promising pharmacologic target for the treatment of insulin resistance and diabetes.
Figures


Similar articles
-
Regulation of insulin signaling and glucose transporter 4 (GLUT4) exocytosis by phosphatidylinositol 3,4,5-trisphosphate (PIP3) phosphatase, skeletal muscle, and kidney enriched inositol polyphosphate phosphatase (SKIP).J Biol Chem. 2012 Mar 2;287(10):6991-9. doi: 10.1074/jbc.M111.335539. Epub 2012 Jan 15. J Biol Chem. 2012. PMID: 22247557 Free PMC article.
-
Phosphatidylinositol 3,4,5-Trisphosphate Phosphatase SKIP Links Endoplasmic Reticulum Stress in Skeletal Muscle to Insulin Resistance.Mol Cell Biol. 2015 Oct 19;36(1):108-18. doi: 10.1128/MCB.00921-15. Print 2016 Jan 1. Mol Cell Biol. 2015. PMID: 26483413 Free PMC article.
-
Regulation of insulin signaling in skeletal muscle by PIP3 phosphatase, SKIP, and endoplasmic reticulum molecular chaperone glucose-regulated protein 78.Biochim Biophys Acta. 2015 Dec;1853(12):3192-201. doi: 10.1016/j.bbamcr.2015.09.009. Epub 2015 Sep 14. Biochim Biophys Acta. 2015. PMID: 26376412
-
Lipid phosphatases as a possible therapeutic target in cases of type 2 diabetes and obesity.Pharmacol Ther. 2006 Dec;112(3):799-809. doi: 10.1016/j.pharmthera.2006.06.001. Epub 2006 Jul 13. Pharmacol Ther. 2006. PMID: 16842857 Review.
-
Metabolism and insulin signaling in common metabolic disorders and inherited insulin resistance.Dan Med J. 2014 Jul;61(7):B4890. Dan Med J. 2014. PMID: 25123125 Review.
Cited by
-
Increased body mass index is linked to systemic inflammation through altered chromatin co-accessibility in human preadipocytes.Nat Commun. 2023 Jul 14;14(1):4214. doi: 10.1038/s41467-023-39919-y. Nat Commun. 2023. PMID: 37452040 Free PMC article.
-
Regulation of insulin signaling and glucose transporter 4 (GLUT4) exocytosis by phosphatidylinositol 3,4,5-trisphosphate (PIP3) phosphatase, skeletal muscle, and kidney enriched inositol polyphosphate phosphatase (SKIP).J Biol Chem. 2012 Mar 2;287(10):6991-9. doi: 10.1074/jbc.M111.335539. Epub 2012 Jan 15. J Biol Chem. 2012. PMID: 22247557 Free PMC article.
-
Skeletal muscle hypertrophy and regeneration: interplay between the myogenic regulatory factors (MRFs) and insulin-like growth factors (IGFs) pathways.Cell Mol Life Sci. 2013 Nov;70(21):4117-30. doi: 10.1007/s00018-013-1330-4. Epub 2013 Apr 4. Cell Mol Life Sci. 2013. PMID: 23552962 Free PMC article. Review.
-
Over-expression of LYRM1 inhibits glucose transport in rat skeletal muscles via attenuated phosphorylation of PI3K (p85) and Akt.Mol Cell Biochem. 2011 Feb;348(1-2):149-54. doi: 10.1007/s11010-010-0649-5. Epub 2010 Nov 12. Mol Cell Biochem. 2011. PMID: 21072680
-
Sac3 is an insulin-regulated phosphatidylinositol 3,5-bisphosphate phosphatase: gain in insulin responsiveness through Sac3 down-regulation in adipocytes.J Biol Chem. 2009 Sep 4;284(36):23961-71. doi: 10.1074/jbc.M109.025361. Epub 2009 Jul 3. J Biol Chem. 2009. PMID: 19578118 Free PMC article.
References
-
- Abel, E. D., O. Peroni, J. K. Kim, Y. B. Kim, O. Boss, E. Hadre, T. Minnemann, G. I. Shulman, and B. B. Kahn. 2001. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 409729-733. - PubMed
-
- Bradley, A. 1987. Production and analysis of chimeric mice, p. 113-151. In E. Robertson (ed.), Teratocarcinomas and embryonic stem cells—a practical approach. IRL Press, Oxford, United Kingdom.
-
- Clement, S., U. Krause, F. Desmedt, J. F. Tanti, J. Befrends, X. Pesesse, T. Sasaki, J. Penninger, M. Doherty, W. Malaisse, J. E. Dumont, Y. Le Marchand-Brustel, C. Euneux, L. Hue, and S. Shurmans. 2001. The lipid phosphatase SHIP2 controls insulin sensitivity. Nature 40992-97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
