Essential role of the response regulator Rrp2 in the infectious cycle of Borrelia burgdorferi
- PMID: 18573895
- PMCID: PMC2519420
- DOI: 10.1128/IAI.00467-08
Essential role of the response regulator Rrp2 in the infectious cycle of Borrelia burgdorferi
Abstract
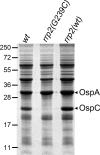
Alteration of surface lipoprotein profiles is a key strategy that the Lyme disease pathogen, Borrelia burgdorferi, has evolved to be maintained within its enzootic cycle between arthropods and mammals. Accumulated evidence indicates that the central regulatory pathway controlling differential gene expression by B. burgdorferi is the RpoN-RpoS pathway (the sigma(54)-sigma(S) sigma factor cascade). It was previously shown that activation of the RpoN-RpoS pathway is controlled by Rrp2, a two-component response regulator and sigma(54)-dependent transcriptional activator. The role of Rrp2 in the infectious cycle of B. burgdorferi has not been determined heretofore. In this report, we demonstrate that an rrp2 mutant defective in activating sigma(54)-dependent transcription was unable to establish infection in mice, but the rrp2 mutant was capable of surviving within ticks during and after tick feeding. Because the rrp2 mutant was defective in the production of OspC, an outer surface lipoprotein essential for mammalian host infection, we further examined whether the loss of infectivity of the rrp2 mutant was solely due to the inability to produce OspC. While transformation with a shuttle vector carrying ospC under the control of a constitutive flaB promoter restored infection to an ospC mutant in immunodeficient SCID mice, it could not rescue the avirulent phenotype of the rrp2 mutant. These data indicate that, in addition to controlling OspC, Rrp2 controls another factor(s) essential for B. burgdorferi to establish infection in mammals. Furthermore, microarray analyses revealed that 125 and 19 genes were positively and negatively regulated, respectively, by Rrp2, which provides a foundation for future identification of additional Rrp2-dependent virulence determinants in B. burgdorferi.
Figures



Similar articles
-
The oligopeptide ABC transporter OppA4 negatively regulates the virulence factor OspC production of the Lyme disease pathogen.Ticks Tick Borne Dis. 2018 Jul;9(5):1343-1349. doi: 10.1016/j.ttbdis.2018.06.006. Epub 2018 Jun 15. Ticks Tick Borne Dis. 2018. PMID: 29921537 Free PMC article.
-
Role of acetyl-phosphate in activation of the Rrp2-RpoN-RpoS pathway in Borrelia burgdorferi.PLoS Pathog. 2010 Sep 16;6(9):e1001104. doi: 10.1371/journal.ppat.1001104. PLoS Pathog. 2010. PMID: 20862323 Free PMC article.
-
Inactivation of bb0184, which encodes carbon storage regulator A, represses the infectivity of Borrelia burgdorferi.Infect Immun. 2011 Mar;79(3):1270-9. doi: 10.1128/IAI.00871-10. Epub 2010 Dec 20. Infect Immun. 2011. PMID: 21173314 Free PMC article.
-
Gene regulation in Borrelia burgdorferi.Annu Rev Microbiol. 2011;65:479-99. doi: 10.1146/annurev.micro.112408.134040. Annu Rev Microbiol. 2011. PMID: 21801026 Review.
-
Borrelia burgdorferi protein interactions critical for microbial persistence in mammals.Cell Microbiol. 2019 Feb;21(2):e12885. doi: 10.1111/cmi.12885. Epub 2018 Jul 8. Cell Microbiol. 2019. PMID: 29934966 Free PMC article. Review.
Cited by
-
Changes in bacterial growth rate govern expression of the Borrelia burgdorferi OspC and Erp infection-associated surface proteins.J Bacteriol. 2013 Feb;195(4):757-64. doi: 10.1128/JB.01956-12. Epub 2012 Dec 7. J Bacteriol. 2013. PMID: 23222718 Free PMC article.
-
Cyclic di-GMP modulates gene expression in Lyme disease spirochetes at the tick-mammal interface to promote spirochete survival during the blood meal and tick-to-mammal transmission.Infect Immun. 2015 Aug;83(8):3043-60. doi: 10.1128/IAI.00315-15. Epub 2015 May 18. Infect Immun. 2015. PMID: 25987708 Free PMC article.
-
A manganese transporter, BB0219 (BmtA), is required for virulence by the Lyme disease spirochete, Borrelia burgdorferi.Proc Natl Acad Sci U S A. 2009 Mar 3;106(9):3449-54. doi: 10.1073/pnas.0812999106. Epub 2009 Feb 13. Proc Natl Acad Sci U S A. 2009. PMID: 19218460 Free PMC article.
-
HrpA, an RNA helicase involved in RNA processing, is required for mouse infectivity and tick transmission of the Lyme disease spirochete.PLoS Pathog. 2013;9(12):e1003841. doi: 10.1371/journal.ppat.1003841. Epub 2013 Dec 19. PLoS Pathog. 2013. PMID: 24367266 Free PMC article.
-
PlzA is a bifunctional c-di-GMP biosensor that promotes tick and mammalian host-adaptation of Borrelia burgdorferi.PLoS Pathog. 2021 Jul 15;17(7):e1009725. doi: 10.1371/journal.ppat.1009725. eCollection 2021 Jul. PLoS Pathog. 2021. PMID: 34265024 Free PMC article.
References
-
- Burgdorfer, W., A. G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwaldt, and J. P. Davis. 1982. Lyme disease, a tick-borne spirochetosis? Science 2161317-1319. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

