TAK1 is required for the survival of hematopoietic cells and hepatocytes in mice
- PMID: 18573910
- PMCID: PMC2442639
- DOI: 10.1084/jem.20080297
TAK1 is required for the survival of hematopoietic cells and hepatocytes in mice
Abstract
Transforming growth factor beta-activated kinase 1 (TAK1), a member of the MAPKKK family, is a key mediator of proinflammatory and stress signals. Activation of TAK1 by proinflammatory cytokines and T and B cell receptors induces the nuclear localization of nuclear factor kappaB (NF-kappaB) and the activation of c-Jun N-terminal kinase (JNK)/AP1 and P38, which play important roles in mediating inflammation, immune responses, T and B cell activation, and epithelial cell survival. Here, we report that TAK1 is critical for the survival of both hematopoietic cells and hepatocytes. Deletion of TAK1 results in bone marrow (BM) and liver failure in mice due to the massive apoptotic death of hematopoietic cells and hepatocytes. Hematopoietic stem cells and progenitors were among those hematopoietic cells affected by TAK1 deletion-induced cell death. This apoptotic cell death is autonomous, as demonstrated by reciprocal BM transplantation. Deletion of TAK1 resulted in the inactivation of both JNK and NF-kappaB signaling, as well as the down-regulation of expression of prosurvival genes.
Figures
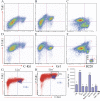
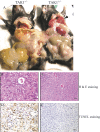


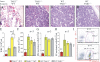
References
-
- Orelio, C., and E. Dzierzak. 2007. Bcl-2 expression and apoptosis in the regulation of hematopoietic stem cells. Leuk. Lymphoma. 48:16–24. - PubMed
-
- Motoyama, N., F. Wang, K.A. Roth, H. Sawa, K. Nakayama, K. Nakayama, I. Negishi, S. Senju, Q. Zhang, S. Fujii, et al. 1995. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science. 267:1506–1510. - PubMed
-
- Opferman, J.T., H. Iwasaki, C.C. Ong, H. Suh, S. Mizuno, K. Akashi, and S.J. Korsmeyer. 2005. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 307:1101–1104. - PubMed
-
- Brandt, J.E., K. Bhalla, and R. Hoffman. 1994. Effects of interleukin-3 and c-kit ligand on the survival of various classes of human hematopoietic progenitor cells. Blood. 83:1507–1514. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

