Chibby cooperates with 14-3-3 to regulate beta-catenin subcellular distribution and signaling activity
- PMID: 18573912
- PMCID: PMC2442201
- DOI: 10.1083/jcb.200709091
Chibby cooperates with 14-3-3 to regulate beta-catenin subcellular distribution and signaling activity
Abstract
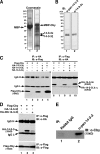
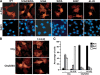
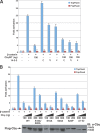
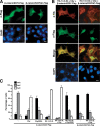
beta-Catenin functions in both cell-cell adhesion and as a transcriptional coactivator in the canonical Wnt pathway. Nuclear accumulation of beta-catenin is the hallmark of active Wnt signaling and is frequently observed in human cancers. Although beta-catenin shuttles in and out of the nucleus, the molecular mechanisms underlying its translocation remain poorly understood. Chibby (Cby) is an evolutionarily conserved molecule that inhibits beta-catenin-mediated transcriptional activation. Here, we identified 14-3-3epsilon and 14-3-3zeta as Cby-binding partners using affinity purification/mass spectrometry. 14-3-3 proteins specifically recognize serine 20 within the 14-3-3-binding motif of Cby when phosphorylated by Akt kinase. Notably, 14-3-3 binding results in sequestration of Cby into the cytoplasm. Moreover, Cby and 14-3-3 form a stable tripartite complex with beta-catenin, causing beta-catenin to partition into the cytoplasm. Our results therefore suggest a novel paradigm through which Cby acts in concert with 14-3-3 proteins to facilitate nuclear export of beta-catenin, thereby antagonizing beta-catenin signaling.
Figures



Similar articles
-
Nuclear-cytoplasmic shuttling of Chibby controls beta-catenin signaling.Mol Biol Cell. 2010 Jan 15;21(2):311-22. doi: 10.1091/mbc.e09-05-0437. Epub 2009 Nov 25. Mol Biol Cell. 2010. PMID: 19940019 Free PMC article.
-
Fine-tuning of nuclear-catenin by Chibby and 14-3-3.Cell Cycle. 2009 Jan 15;8(2):210-3. doi: 10.4161/cc.8.2.7394. Epub 2009 Jan 12. Cell Cycle. 2009. PMID: 19158508 Free PMC article. Review.
-
Structural Analysis of the 14-3-3ζ/Chibby Interaction Involved in Wnt/β-Catenin Signaling.PLoS One. 2015 Apr 24;10(4):e0123934. doi: 10.1371/journal.pone.0123934. eCollection 2015. PLoS One. 2015. PMID: 25909186 Free PMC article.
-
Chibby forms a homodimer through a heptad repeat of leucine residues in its C-terminal coiled-coil motif.BMC Mol Biol. 2009 May 12;10:41. doi: 10.1186/1471-2199-10-41. BMC Mol Biol. 2009. PMID: 19435523 Free PMC article.
-
Looking beyond the Wnt pathway for the deep nature of β-catenin.EMBO Rep. 2013 May;14(5):422-33. doi: 10.1038/embor.2013.45. Epub 2013 Apr 19. EMBO Rep. 2013. PMID: 23598517 Free PMC article. Review.
Cited by
-
14-3-3σ regulates β-catenin-mediated mouse embryonic stem cell proliferation by sequestering GSK-3β.PLoS One. 2012;7(6):e40193. doi: 10.1371/journal.pone.0040193. Epub 2012 Jun 29. PLoS One. 2012. PMID: 22768254 Free PMC article.
-
Nuclear-cytoplasmic shuttling of Chibby controls beta-catenin signaling.Mol Biol Cell. 2010 Jan 15;21(2):311-22. doi: 10.1091/mbc.e09-05-0437. Epub 2009 Nov 25. Mol Biol Cell. 2010. PMID: 19940019 Free PMC article.
-
Opposing roles of ICAT and Wnt/β-catenin signaling in NSC67657-induced monocytic differentiation.Oncotarget. 2017 Jul 22;8(41):69924-69933. doi: 10.18632/oncotarget.19457. eCollection 2017 Sep 19. Oncotarget. 2017. PMID: 29050252 Free PMC article.
-
Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action.Am J Psychiatry. 2010 Apr;167(4):388-96. doi: 10.1176/appi.ajp.2009.08121873. Epub 2009 Nov 16. Am J Psychiatry. 2010. PMID: 19917593 Free PMC article. Review.
-
Cby1 promotes Ahi1 recruitment to a ring-shaped domain at the centriole-cilium interface and facilitates proper cilium formation and function.Mol Biol Cell. 2014 Oct 1;25(19):2919-33. doi: 10.1091/mbc.E14-02-0735. Epub 2014 Aug 7. Mol Biol Cell. 2014. PMID: 25103236 Free PMC article.
References
-
- Aitken, A. 2006. 14-3-3 proteins: a historic overview. Semin. Cancer Biol. 16:162–172. - PubMed
-
- Andjelkovic, M., D.R. Alessi, R. Meier, A. Fernandez, N.J.C. Lamb, M. Frech, P. Cron, P. Cohen, J.M. Lucocq, and B.A. Hemmings. 1997. J. Biol. Chem. 272:31515–31524. - PubMed
-
- Borgatti, P., A.M. Martelli, G. Tabellini, A. Bellacosa, S. Capitani, and L.M. Neri. 2003. Threonine 308 phosphorylated form of Akt translocates to the nucleus of PC12 cells under nerve growth factor stimulation and associate with the nuclear matrix protein nucleolin. J. Cell. Physiol. 196:79–88. - PubMed
-
- Brazil, D.P., J. Park, and B.A. Hemmings. 2002. PKB binding proteins. Getting in on the Akt. Cell. 111:293–303. - PubMed
-
- Brunet, A., A. Bonni, M.J. Zigmond, M.Z. Lin, P. Juo, L.S. Hu, M.J. Anderson, K.C. Arden, J. Blenis, and M.E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 96:857–868. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

