Chronic administration of tenofovir to rhesus macaques from infancy through adulthood and pregnancy: summary of pharmacokinetics and biological and virological effects
- PMID: 18573931
- PMCID: PMC2533487
- DOI: 10.1128/AAC.00350-08
Chronic administration of tenofovir to rhesus macaques from infancy through adulthood and pregnancy: summary of pharmacokinetics and biological and virological effects
Abstract
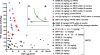
The reverse transcriptase (RT) inhibitor tenofovir (TFV) is highly effective in the simian immunodeficiency virus (SIV) macaque model of human immunodeficiency virus infection. The current report describes extended safety and efficacy data on 32 animals that received prolonged (>or=1- to 13-year) daily subcutaneous TFV regimens. The likelihood of renal toxicity (proximal renal tubular dysfunction [PRTD]) correlated with plasma drug concentrations, which depended on the dosage regimen and age-related changes in drug clearance. Below a threshold area under the concentration-time curve for TFV in plasma of approximately 10 microg x h/ml, an exposure severalfold higher than that observed in humans treated orally with 300 mg TFV disoproxil fumarate (TDF), prolonged TFV administration was not associated with PRTD based on urinalysis, serum chemistry analyses, bone mineral density, and clinical observations. At low-dose maintenance regimens, plasma TFV concentrations and intracellular TFV diphosphate concentrations were similar to or slightly higher than those observed in TDF-treated humans. No new toxicities were identified. The available evidence does not suggest teratogenic effects of prolonged low-dose TFV treatment; by the age of 10 years, one macaque, on TFV treatment since birth, had produced three offspring that were healthy by all criteria up to the age of 5 years. Despite the presence of viral variants with a lysine-to-arginine substitution at codon 65 (K65R) of RT in all 28 SIV-infected animals, 6 animals suppressed viremia to undetectable levels for as long as 12 years of TFV monotherapy. In conclusion, these findings illustrate the safety and sustained benefits of prolonged TFV-containing regimens throughout development from infancy to adulthood, including pregnancy.
Figures



References
-
- AIDS Vaccine Advocacy Coalition. March 2005, posting date. Will a pill a day prevent HIV? Anticipating the results of the tenofovir “PREP” trials. http://avac.org/pdf/tenofovir.pdf.
-
- Alatrakchi, N., C. Duvivier, D. Costagliola, A. Samri, A. Marcelin, G. Kamkamidze, M. Astriti, R. Agher, V. Calvez, B. Autran, and C. Katlama. 2005. Persistent low viral load on antiretroviral therapy is associated with T cell-mediated control of HIV replication. AIDS 19:25-33. - PubMed
-
- Antiretroviral Pregnancy Registry Steering Committee. 2007. Antiretroviral Pregnancy Registry interim report for 1 January 1989 through 31 July 2007. Registry Coordinating Center, Wilmington, NC.
-
- Barditch-Crovo, P., S. G. Deeks, A. Collier, S. Safrin, D. F. Coakley, M. Miller, B. P. Kearney, R. L. Coleman, P. D. Lamy, J. O. Kahn, I. McGowan, and P. S. Lietman. 2001. Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 45:2733-2739. - PMC - PubMed
-
- Bedard, J., S. May, M. Lis, L. Tryphonas, J. Drach, J. Huffman, R. Sidwell, L. Chan, T. Bowlin, and R. Rando. 1999. Comparative study of the anti-human cytomegalovirus activities and toxicities of a tetrahydrofuran phosphonate analogue of guanosine and cidofovir. Antimicrob. Agents Chemother. 43:557-567. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

