Transient blockade of the inducible costimulator pathway generates long-term tolerance to factor VIII after nonviral gene transfer into hemophilia A mice
- PMID: 18574023
- PMCID: PMC2518877
- DOI: 10.1182/blood-2008-01-128413
Transient blockade of the inducible costimulator pathway generates long-term tolerance to factor VIII after nonviral gene transfer into hemophilia A mice
Abstract
Formation of inhibitory antibodies is a common problem encountered in clinical treatment for hemophilia. Human factor VIII (hFVIII) plasmid gene therapy in hemophilia A mice also leads to strong humoral responses. We demonstrate that short-term therapy with an anti-ICOS monoclonal antibody to transiently block the inducible costimulator/inducible costimulator ligand (ICOS/ICOSL) signaling pathway led to sustained tolerance to hFVIII in hFVIII plasmid-treated hemophilia A mice and allowed persistent, high-level FVIII functional activity (100%-300% of normal). Anti-ICOS treatment resulted in depletion of ICOS(+)CD4(+) T cells and activation of CD25(+)Foxp3(+) Tregs in the peripheral blood, spleen, and lymph nodes. CD4(+) T cells from anti-ICOS-treated mice did not proliferate in response to hFVIII stimulation and produced high levels of regulatory cytokines, including interleukin-10 and transforming growth factor-beta. Moreover, CD4(+)CD25(+) Tregs from tolerized mice adoptively transferred dominant tolerance in syngeneic hFVIII plasmid-treated hemophilia A mice and reduced the production of antibodies against FVIII. Anti-ICOS-treated mice tolerized to hFVIII generated normal primary and secondary antibody responses after immunization with the T-dependent antigen, bacteriophage Phix 174, indicating maintenance of immune competency. Our data indicate that transient anti-ICOS monoclonal antibody treatment represents a novel single-agent immunomodulatory strategy to overcome the immune responses against transgene product after gene therapy.
Figures



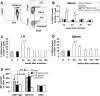
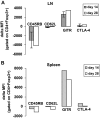
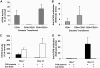

References
-
- Fields PA, Kowalczyk DW, Arruda VR, et al. Role of vector in activation of T cell subsets in immune responses against the secreted transgene product factor IX. Mol Ther. 2000;1:225–235. - PubMed
-
- Chao H, Mao L, Bruce AT, Walsh CE. Sustained expression of human factor VIII in mice using a parvovirus-based vector. Blood. 2000;95:1594–1599. - PubMed
-
- Greengard JS, Jolly PJ. Animal testing of retroviral-mediated gene therapy for factor VIII deficiency. Thromb Haemost. 1999;82:555–561. - PubMed
-
- Ye P, Thompson AR, Sarkar R, et al. Naked DNA transfer of Factor VIII induced transgene-specific, species-independent immune response in hemophilia A mice. Mol Ther. 2004;10:117–126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

