Angiotensin II-triggered p44/42 mitogen-activated protein kinase mediates sympathetic excitation in heart failure rats
- PMID: 18574076
- PMCID: PMC2785116
- DOI: 10.1161/HYPERTENSIONAHA.108.110445
Angiotensin II-triggered p44/42 mitogen-activated protein kinase mediates sympathetic excitation in heart failure rats
Abstract
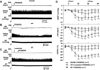
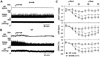
Angiotensin II (Ang II), acting via angiotensin type 1 receptors in the brain, activates the sympathetic nervous system in heart failure (HF). We reported recently that Ang II stimulates mitogen-activated protein kinase (MAPK) to upregulate brain angiotensin type 1 receptors in HF rats. In this study we tested the hypothesis that Ang II-activated MAPK signaling pathways contribute to sympathetic excitation in HF. Intracerebroventricular administration of PD98059 and UO126, 2 selective p44/42 MAPK inhibitors, induced significant decreases in mean arterial pressure, heart rate, and renal sympathetic nerve activity in HF rats, but had no effect on these variables in sham-operated rats. Pretreatment with losartan attenuated the effects of PD98059. Intracerebroventricular administration of the p38 MAPK inhibitor SB203580 and the c-Jun N-terminal kinase inhibitor SP600125 had no effect on mean arterial pressure, heart rate, or renal sympathetic nerve activity in HF. The phosphatidylinositol 3-kinase inhibitor LY294002 induced a small decrease in mean arterial pressure and heart rate but no change in renal sympathetic nerve activity. Immunofluorescent staining demonstrated increased p44/42 MAPK activity in neurons of the paraventricular nucleus of the hypothalamus of HF rats, colocalized with Fra-like activity (indicating chronic neuronal excitation). Intracerebroventricular PD98059 and UO126 reduced Fra-like activity in the paraventricular nucleus of the hypothalamus neurons in HF rats. In confirmatory acute studies, intracerebroventricular Ang II increased mean arterial pressure, heart rate, and renal sympathetic nerve activity in baroreceptor-denervated rats and Fra-like immunoreactivity in the paraventricular nucleus of the hypothalamus of neurally intact rats. Central administration of PD98059 markedly reduced these responses. These data demonstrate that intracellular p44/42 MAPK activity contributes to Ang II-induced neuronal excitation in the paraventricular nucleus of the hypothalamus and augmented sympathetic nerve activity in rats with HF.
Figures





References
-
- Cohn JN. Abnormalities of peripheral sympathetic nervous system control in congestive heart failure. Circulation. 1990;82:I59–I67. - PubMed
-
- Francis J, Weiss RM, Wei SG, Johnson AK, Felder RB. Progression of heart failure after myocardial infarction in the rat. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1734–R1745. - PubMed
-
- Packer M. The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J Am Coll Cardiol. 1992;20:248–254. - PubMed
-
- Pinto YM, Buikema H, van Gilst WH, Lie KI. Activated tissue renin-angiotensin systems add to the progression of heart failure. Basic Res Cardiol. 1996;91 Suppl 2:85–90. - PubMed
-
- Francis J, Wei SG, Weiss RM, Felder RB. Brain angiotensin-converting enzyme activity and autonomic regulation in heart failure. Am J Physiol Endocrinol Metab. 2004;287:H2138–H2146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

