A mutant small heat shock protein with increased thylakoid association provides an elevated resistance against UV-B damage in synechocystis 6803
- PMID: 18574246
- PMCID: PMC2516980
- DOI: 10.1074/jbc.M710400200
A mutant small heat shock protein with increased thylakoid association provides an elevated resistance against UV-B damage in synechocystis 6803
Abstract
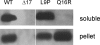
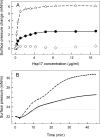
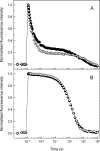
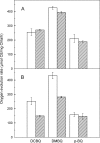
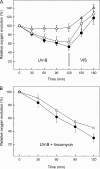
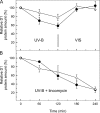
Besides acting as molecular chaperones, the amphitropic small heat shock proteins (sHsps) are suggested to play an additional role in membrane quality control. We investigated sHsp membrane function in the model cyanobacterium Synechocystis sp. PPC 6803 using mutants of the single sHsp from this organism, Hsp17. We examined mutants in the N-terminal arm, L9P and Q16R, for altered interaction with thylakoid and lipid membranes and examined the effects of these mutations on thylakoid functions. These mutants are unusual in that they retain their oligomeric state and chaperone activity in vitro but fail to confer thermotolerance in vivo. We found that both mutant proteins had dramatically altered membrane/lipid interaction properties. Whereas L9P showed strongly reduced binding to thylakoid and model membranes, Q16R was almost exclusively membrane-associated, properties that may be the cause of reduced heat tolerance of cells carrying these mutations. Among the lipid classes tested, Q16R displayed the highest interaction with negatively charged SQDG. In Q16R cells a specific alteration of the thylakoid-embedded Photosystem II (PSII) complex was observed. Namely, the binding of plastoquinone and quinone analogue acceptors to the Q(B) site was modified. In addition, the presence of Q16R dramatically reduced UV-B damage of PSII activity because of enhanced PSII repair. We suggest these effects occur at least partly because of increased interaction of Q16R with SQDG in the PSII complex. Our findings further support the model that membrane association is a functional property of sHsps and suggest sHsps as a possible biotechnological tool to enhance UV protection of photosynthetic organisms.
Figures

Similar articles
-
Synechocystis HSP17 is an amphitropic protein that stabilizes heat-stressed membranes and binds denatured proteins for subsequent chaperone-mediated refolding.Proc Natl Acad Sci U S A. 2001 Mar 13;98(6):3098-103. doi: 10.1073/pnas.051619498. Epub 2001 Feb 27. Proc Natl Acad Sci U S A. 2001. PMID: 11248038 Free PMC article.
-
Thylakoid membrane lipid sulfoquinovosyl-diacylglycerol (SQDG) is required for full functioning of photosystem II in Thermosynechococcus elongatus.J Biol Chem. 2018 Sep 21;293(38):14786-14797. doi: 10.1074/jbc.RA118.004304. Epub 2018 Aug 3. J Biol Chem. 2018. PMID: 30076221 Free PMC article.
-
"Heat shock lipid" in cyanobacteria during heat/light-acclimation.Arch Biochem Biophys. 2005 Apr 15;436(2):346-54. doi: 10.1016/j.abb.2005.02.018. Arch Biochem Biophys. 2005. PMID: 15797247
-
Close Relationships Between the PSII Repair Cycle and Thylakoid Membrane Dynamics.Plant Cell Physiol. 2016 Jun;57(6):1115-22. doi: 10.1093/pcp/pcw050. Epub 2016 Mar 26. Plant Cell Physiol. 2016. PMID: 27017619 Review.
-
Is the lipochaperone activity of sHSP a key to the stress response encoded in its primary sequence?Cell Stress Chaperones. 2023 Jan;28(1):21-33. doi: 10.1007/s12192-022-01308-7. Epub 2022 Nov 11. Cell Stress Chaperones. 2023. PMID: 36367671 Free PMC article. Review.
Cited by
-
The Small Heat Shock Protein, HSPB1, Interacts with and Modulates the Physical Structure of Membranes.Int J Mol Sci. 2022 Jun 30;23(13):7317. doi: 10.3390/ijms23137317. Int J Mol Sci. 2022. PMID: 35806322 Free PMC article.
-
The Common Bean Small Heat Shock Protein Nodulin 22 from Phaseolus vulgaris L. Assembles into Functional High-Molecular-Weight Oligomers.Molecules. 2022 Dec 8;27(24):8681. doi: 10.3390/molecules27248681. Molecules. 2022. PMID: 36557819 Free PMC article.
-
Translation on demand by a simple RNA-based thermosensor.Nucleic Acids Res. 2011 Apr;39(7):2855-68. doi: 10.1093/nar/gkq1252. Epub 2010 Dec 3. Nucleic Acids Res. 2011. PMID: 21131278 Free PMC article.
-
Small heat shock protein Hsp17.8 functions as an AKR2A cofactor in the targeting of chloroplast outer membrane proteins in Arabidopsis.Plant Physiol. 2011 Sep;157(1):132-46. doi: 10.1104/pp.111.178681. Epub 2011 Jul 5. Plant Physiol. 2011. PMID: 21730198 Free PMC article.
-
The Caenorhabditis elegans 12-kDa small heat shock proteins with little in vitro chaperone activity play crucial roles for its dauer formation, longevity, and reproduction.Protein Sci. 2021 Oct;30(10):2170-2182. doi: 10.1002/pro.4160. Epub 2021 Jul 31. Protein Sci. 2021. PMID: 34272907 Free PMC article.
References
-
- van Montfort, R. L. M., Basha, E., Friedrich, K. L., Slingsby, C., and Vierling, E. (2001) Nat. Struct. Biol. 8 1025–1030 - PubMed
-
- De Jong, W. W., Caspers, G.-J., and Leunissen, J. A. M. (1998) Int. J. Biol. Macromol. 22 151–162 - PubMed
-
- Waters, E. R., Lee, G. J., and Vierling, E. (1996) J. Exp. Bot. 47 325–338
-
- Stamler, R., Kappe, G., Boelens, W., and Slingsby, C. (2005) J. Mol. Biol. 353 68–79 - PubMed
-
- Basha, E., Lee, G. J., Breci, L. A., Hausrath, A. C., Buan, N. R., Giese, K. C., and Vierling, E. (2004) J. Biol. Chem. 279 7566–7575 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

