High-affinity blockade of voltage-operated skeletal muscle and neuronal sodium channels by halogenated propofol analogues
- PMID: 18574460
- PMCID: PMC2538694
- DOI: 10.1038/bjp.2008.255
High-affinity blockade of voltage-operated skeletal muscle and neuronal sodium channels by halogenated propofol analogues
Abstract
Background and purpose: Voltage-operated sodium channels constitute major target sites for local anaesthetic-like action. The clinical use of local anaesthetics is still limited by severe side effects, in particular, arrhythmias and convulsions. These side effects render the search for new local anaesthetics a matter of high interest.
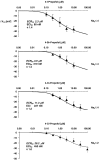
Experimental approach: We have investigated the effects of three halogenated structural analogues of propofol on voltage-operated human skeletal muscle sodium channels (Na(V)1.4) and the effect of one compound (4-chloropropofol) on neuronal sodium channels (Na(V)1.2) heterologously expressed in human embryonic kidney cell line 293.
Key results: 4-Iodo-, 4-bromo- and 4-chloropropofol reversibly suppressed depolarization-induced whole-cell sodium inward currents with high potency. The IC(50) for block of resting channels at -150 mV was 2.3, 3.9 and 11.3 microM in Na(V)1.4, respectively, and 29.2 microM for 4-chloropropofol in Na(V)1.2. Membrane depolarization inducing inactivation strongly increased the blocking potency of all compounds. Estimated affinities for the fast-inactivated channel state were 81 nM, 312 nM and 227 nM for 4-iodopropofol, 4-bromopropofol and 4-chloropropofol in Na(V)1.4, and 450 nM for 4-chloropropofol in Na(V)1.2. Recovery from fast inactivation was prolonged in the presence of drug leading to an accumulation of block during repetitive stimulation at high frequencies (100 Hz).
Conclusions and implications: Halogenated propofol analogues constitute a novel class of sodium channel-blocking drugs possessing almost 100-fold higher potency compared with the local anaesthetic and anti-arrhythmic drug lidocaine. Preferential drug binding to inactivated channel states suggests that halogenated propofol analogues might be especially effective in suppressing ectopic discharges in a variety of pathological conditions.
Figures




References
-
- Amir R, Argoff CE, Bennett GJ, Cummins TR, Durieux ME, Gerner P, et al. The role of sodium channels in chronic inflammatory and neuropathic pain. J Pain. 2006;7:S1–S29. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

