Interferon-inducible antiviral effectors
- PMID: 18575461
- PMCID: PMC2522268
- DOI: 10.1038/nri2314
Interferon-inducible antiviral effectors
Abstract
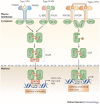
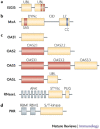
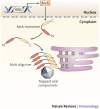
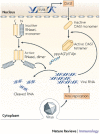
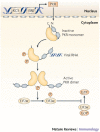
Since the discovery of interferons (IFNs), considerable progress has been made in describing the nature of the cytokines themselves, the signalling components that direct the cell response and their antiviral activities. Gene targeting studies have distinguished four main effector pathways of the IFN-mediated antiviral response: the Mx GTPase pathway, the 2',5'-oligoadenylate-synthetase-directed ribonuclease L pathway, the protein kinase R pathway and the ISG15 ubiquitin-like pathway. As discussed in this Review, these effector pathways individually block viral transcription, degrade viral RNA, inhibit translation and modify protein function to control all steps of viral replication. Ongoing research continues to expose additional activities for these effector proteins and has revealed unanticipated functions of the antiviral response.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

