Effects of ferumoxides-protamine sulfate labeling on immunomodulatory characteristics of macrophage-like THP-1 cells
- PMID: 18575575
- PMCID: PMC2423478
- DOI: 10.1371/journal.pone.0002499
Effects of ferumoxides-protamine sulfate labeling on immunomodulatory characteristics of macrophage-like THP-1 cells
Abstract
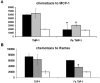
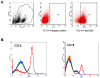
Superparamagnetic Iron Oxide (SPIO) complexed with cationic transfection agent is used to label various mammalian cells. Labeled cells can then be utilized as an in vivo magnetic resonance imaging (MRI) probes. However, certain number of in vivo administered labeled cells may be cleared from tissues by the host's macrophages. For successful translation to routine clinical application of SPIO labeling method it is important that this mode of in vivo clearance of iron does not elicit any diverse immunological effects. The purpose of this study was to demonstrate that SPIO agent ferumoxides-protamine sulfate (FePro) incorporation into macrophages does not alter immunological properties of these cells with regard to differentiation, chemotaxis, and ability to respond to the activation stimuli and to modulate T cell response. We used THP-1 cell line as a model for studying macrophage cell type. THP-1 cells were magnetically labeled with FePro, differentiated with 100 nM of phorbol ester, 12-Myristate-13-acetate (TPA) and stimulated with 100 ng/ml of LPS. The results showed 1) FePro labeling had no effect on the changes in morphology and expression of cell surface proteins associated with TPA induced differentiation; 2) FePro labeled cells responded to LPS with slightly higher levels of NFkappaB pathway activation, as shown by immunobloting; TNF-alpha secretion and cell surface expression levels of CD54 and CD83 activation markers, under these conditions, were still comparable to the levels observed in non-labeled cells; 3) FePro labeling exhibited differential, chemokine dependent, effect on THP-1 chemotaxis with a decrease in cell directional migration to MCP-1; 4) FePro labeling did not affect the ability of THP-1 cells to down-regulate T cell expression of CD4 and CD8 and to induce T cell proliferation. Our study demonstrated that intracellular incorporation of FePro complexes does not alter overall immunological properties of THP-1 cells. The described experiments provide the model for studying the effects of in vivo clearance of iron particles via incorporation into the host's macrophages that may follow after in vivo application of any type of magnetically labeled mammalian cells. To better mimic the complex in vivo scenario, this model may be further exploited by introducing additional cellular and biological, immunologically relevant, components.
Conflict of interest statement
Figures




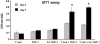
References
-
- Ferrucci JT, Stark DD. Iron oxide-enhanced MR imaging of the liver and spleen: review of the first 5 years. AJR Am J Roentgenol. 1990;155:943–950. - PubMed
-
- Matuszewski L, Persigehl T, Wall A, Schwindt W, Tombach B, et al. Cell tagging with clinically approved iron oxides: feasibility and effect of lipofection, particle size, and surface coating on labeling efficiency. Radiology. 2005;235:155–161. - PubMed
-
- Dodd CH, Hsu HC, Chu WJ, Yang P, Zhang HG, et al. Normal T-cell response and in vivo magnetic resonance imaging of T cells loaded with HIV transactivator-peptide-derived superparamagnetic nanoparticles. J Immunol Methods. 2001;256:89–105. - PubMed
-
- Ahrens ET, Feili-Hariri M, Xu H, Genove G, Morel PA. Receptor-mediated endocytosis of iron-oxide particles provides efficient labeling of dendritic cells for in vivo MR imaging. Magn Reson Med. 2003;49:1006–1013. - PubMed
-
- Lewin M, Carlesso N, Tung CH, Tang XW, Cory D, et al. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol. 2000;18:410–414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

