Climate extremes promote fatal co-infections during canine distemper epidemics in African lions
- PMID: 18575601
- PMCID: PMC2435602
- DOI: 10.1371/journal.pone.0002545
Climate extremes promote fatal co-infections during canine distemper epidemics in African lions
Abstract
Extreme climatic conditions may alter historic host-pathogen relationships and synchronize the temporal and spatial convergence of multiple infectious agents, triggering epidemics with far greater mortality than those due to single pathogens. Here we present the first data to clearly illustrate how climate extremes can promote a complex interplay between epidemic and endemic pathogens that are normally tolerated in isolation, but with co-infection, result in catastrophic mortality. A 1994 canine distemper virus (CDV) epidemic in Serengeti lions (Panthera leo) coincided with the death of a third of the population, and a second high-mortality CDV epidemic struck the nearby Ngorongoro Crater lion population in 2001. The extent of adult mortalities was unusual for CDV and prompted an investigation into contributing factors. Serological analyses indicated that at least five "silent" CDV epidemics swept through the same two lion populations between 1976 and 2006 without clinical signs or measurable mortality, indicating that CDV was not necessarily fatal. Clinical and pathology findings suggested that hemoparsitism was a major contributing factor during fatal epidemics. Using quantitative real-time PCR, we measured the magnitude of hemoparasite infections in these populations over 22 years and demonstrated significantly higher levels of Babesia during the 1994 and 2001 epidemics. Babesia levels correlated with mortalities and extent of CDV exposure within prides. The common event preceding the two high mortality CDV outbreaks was extreme drought conditions with wide-spread herbivore die-offs, most notably of Cape buffalo (Syncerus caffer). As a consequence of high tick numbers after the resumption of rains and heavy tick infestations of starving buffalo, the lions were infected by unusually high numbers of Babesia, infections that were magnified by the immunosuppressive effects of coincident CDV, leading to unprecedented mortality. Such mass mortality events may become increasingly common if climate extremes disrupt historic stable relationships between co-existing pathogens and their susceptible hosts.
Conflict of interest statement
Figures

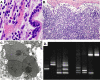
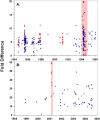

References
-
- Stokstad E. Genomics. Puzzling decline of U.S.bees linked to virus from Australia. Science. 2007;317:1304–1305. - PubMed
-
- Harvell CD, Mitchell CE, Ward JR, Altizer S, Dobson AP, et al. Climate warming and disease risks for terrestrial and marine biota. Science. 2002;296:2158–2162. - PubMed
-
- Berger L, Speare R, Hines HB. Effect of season and temperature on mortality in amphibians due to chytridiomycosis. Aust Vet J. 2004;82:434–439. - PubMed
-
- Harvell CD, Kim K, Burkholder JM, Cowell RR, Epstein PR, et al. Emerging marine diseases-Climate links and anthropogenic factors. Science. 1999;285:1505–1510. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

