Mesenchymal stem cells in early entry of breast cancer into bone marrow
- PMID: 18575622
- PMCID: PMC2430536
- DOI: 10.1371/journal.pone.0002563
Mesenchymal stem cells in early entry of breast cancer into bone marrow
Abstract
Background: An understanding of BC cell (BCC) entry into bone marrow (BM) at low tumor burden is limited when compared to highly metastatic events during heavy tumor burden. BCCs can achieve quiescence, without interfering with hematopoiesis. This occurs partly through the generation of gap junctions with BM stroma, located close to the endosteum. These events are partly mediated by the evolutionary conserved gene, Tac1.
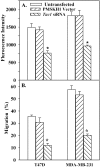
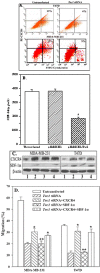
Methodology/principal findings: This study focuses on the role of mesenchymal stem cells (MSCs), Tac1, SDF-1 and CXCR4 in BCC entry into BM. The model is established in studies with low numbers of tumor cells, and focuses on cancer cells with low metastatic and invasion potential. This allowed us to recapitulate early event, and to study cancer cells with low invasive potential, even when they are part of larger numbers of highly metastatic cells. A novel migration assay showed a facilitating role of MSCs in BCC migration across BM endothelial cells. siRNA and ectopic expression studies showed a central role for Tac1 and secondary roles for SDF-1alpha and CXCR4. We also observed differences in the mechanisms between low invasive and highly metastatic cells. The in vitro studies were verified in xenogeneic mouse models that showed a preference for low invasive BCCs to BM, but comparable movement to lung and BM by highly metastatic BCCs. The expressions of Tac1 and production of SDF-1alpha were verified in primary BCCs from paired samples of BM aspirates and peripheral blood.
Conclusions/significance: MSC facilitate BCC entry into BM, partly through Tac1-mediated regulation of SDF-1alpha and CXCR4. We propose a particular population of BCC with preference for BM could be isolated for characterization. This population might be the subset that enter BM at an early time period, and could be responsible for cancer resurgence and resistance to current therapies.
Conflict of interest statement
Figures





References
-
- Bigioni M, Benzo A, Irrissuto C, Maggi CA, Goso C. Role of NK-1 and NK-2 tachykinin receptor antagonism on the growth of human breast carcinoma cell line MDA-MB-231. Anticancer Drugs. 2007;16:1083–1089. - PubMed
-
- Rao G, Patel PS, Idler SP, Maloof P, Gascon P, et al. Facilitating role of preprotachykinin-I gene in the integration of breast cancer cells within the stromal compartment of the bone marrow: A model of early cancer progression. Cancer Res. 2004;64:2874–2881. - PubMed
-
- Greco SJ, Corcoran KE, Cho KJ, Rameshwar P. Tachykinins in the emerging immune system: Relevance to bone marrow homeostasis and maintenance of hematopoietic stem cells. Frontiers Biosci. 2004;9:1782–1793. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

