An open label, randomised trial of artesunate+amodiaquine, artesunate+chlorproguanil-dapsone and artemether-lumefantrine for the treatment of uncomplicated malaria
- PMID: 18575626
- PMCID: PMC2430614
- DOI: 10.1371/journal.pone.0002530
An open label, randomised trial of artesunate+amodiaquine, artesunate+chlorproguanil-dapsone and artemether-lumefantrine for the treatment of uncomplicated malaria
Abstract
Background: Artesunate+amodiaquine (AS+AQ) and artemether-lumefantrine (AL) are now the most frequently recommended first line treatments for uncomplicated malaria in Africa. Artesunate+chlorproguanil-dapsone (AS+CD) was a potential alternative for treatment of uncomplicated malaria. A comparison of the efficacy and safety of these three drug combinations was necessary to make evidence based drug treatment policies.
Methods: Five hundred and thirty-four, glucose-6-phosphate dehydrogenase (G6PD) normal children were randomised in blocks of 15 to the AS+AQ, AL or AS+CD groups. Administration of study drugs was supervised by project staff and the children were followed up at r home on days 1,2,3,7,14 and 28 post treatment. Parasitological and clinical failures and adverse events were compared between the study groups.
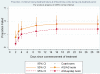
Main findings: In a per-protocol analysis, the parasitological and clinical failure rate at day 28 post treatment (PCF28) was lower in the AS+AQ group compared to the AL or AS+CD groups (corrected for re-infections: 6.6% vs 13.8% and 13.8% respectively, p = 0.08; uncorrected: 14.6% vs 27.6% and 28.1% respectively, p = 0.005). In the intention to treat analysis, the rate of early treatment failure was high in all three groups (AS+AQ 13.3%; AL 15.2%; and AS+CD 9.3%, p = 0.2) primarily due to vomiting. However, the PCF28 corrected for re-infection was lower, though not significantly, in the AS+AQ group compared to the AL or the AS+CD groups (AS+AQ 18.3%; AL 24.2%; AS+CD 20.8%, p = 0.4) The incidence of adverse events was comparable between the groups.
Conclusions: AS+AQ is an appropriate first line treatment for uncomplicated malaria in Ghana and possibly in the neighbouring countries in West Africa. The effectiveness of AL in routine programme conditions needs to be studied further in West Africa.
Trial registration: ClinicalTrials.gov NCT00119145.
Conflict of interest statement
Figures
References
-
- White NJ, Nosten F, Looareesuwan S, Watkins WM, Marsh K, et al. Averting a malaria disaster. Lancet. 1999;353:1965–7. - PubMed
-
- World Health Organisation (WHO) Antimalaria drug combination therarpy: report of a WHO Technical Consultation. Document no. WHO/CDS/RBM/2001.35. Geneva: WHO; 2001.
-
- Martensson A, Stromberg J, Sisowath C, Msellem ML, Gil JP, et al. Efficacy of artesunate plus amodiaquine versus that of artemether-Lumefantrine for the treatment of uncomplicated childhood plasmodium falciparum malaria in Zanzibar, Tanzania. Clinical Infectious Diseases. 2005;41:1079–86. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous