A vaccine against nicotine for smoking cessation: a randomized controlled trial
- PMID: 18575629
- PMCID: PMC2432028
- DOI: 10.1371/journal.pone.0002547
A vaccine against nicotine for smoking cessation: a randomized controlled trial
Abstract
Background: Tobacco dependence is the leading cause of preventable death and disabilities worldwide and nicotine is the main substance responsible for the addiction to tobacco. A vaccine against nicotine was tested in a 6-month randomized, double blind phase II smoking cessation study in 341 smokers with a subsequent 6-month follow-up period.
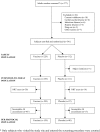
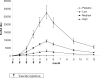
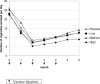
Methodology/principal findings: 229 subjects were randomized to receive five intramuscular injections of the nicotine vaccine and 112 to receive placebo at monthly intervals. All subjects received individual behavioral smoking cessation counseling. The vaccine was safe, generally well tolerated and highly immunogenic, inducing a 100% antibody responder rate after the first injection. Point prevalence of abstinence at month 2 showed a statistically significant difference between subjects treated with Nicotine-Qbeta (47.2%) and placebo (35.1%) (P = 0.036), but continuous abstinence between months 2 and 6 was not significantly different. However, in subgroup analysis of the per-protocol population, the third of subjects with highest antibody levels showed higher continuous abstinence from month 2 until month 6 (56.6%) than placebo treated participants (31.3%) (OR 2.9; P = 0.004) while medium and low antibody levels did not increase abstinence rates. After 12 month, the difference in continuous abstinence rate between subjects on placebo and those with high antibody response was maintained (difference 20.2%, P = 0.012).
Conclusions: Whereas Nicotine-Qbeta did not significantly increase continuous abstinence rates in the intention-to-treat population, subgroup analyses of the per-protocol population suggest that such a vaccination against nicotine can significantly increase continuous abstinence rates in smokers when sufficiently high antibody levels are achieved. Immunotherapy might open a new avenue to the treatment of nicotine addiction.
Trial registration: Swiss Medical Registry 2003DR2327; ClinicalTrials.gov NCT00369616.
Conflict of interest statement
Figures
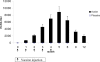

References
-
- Proctor RN. The global smoking epidemic: a history and status report. Clin Lung Cancer. 2004;5:371–376. - PubMed
-
- Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, et al. 2000. Treating Tobacco Use and Dependance: Clinical Practice Guideline (revised 2000)
-
- Hughes J, Stead L, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev CD000031 2004 - PubMed
-
- Hieda Y, Keyler DE, Vandevoort JT, Kane JK, Ross CA, et al. Active immunization alters the plasma nicotine concentration in rats. J Pharmacol Exp Ther. 1997;283:1076–1081. - PubMed
-
- Pentel PR, Malin DH, Ennifar S, Hieda Y, Keyler DE, et al. A nicotine conjugate vaccine reduces nicotine distribution to brain and attenuates its behavioral and cardiovascular effects in rats. Pharmacol Biochem Behav. 2000;65:191–198. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

