The renal cortical interstitium: morphological and functional aspects
- PMID: 18575881
- PMCID: PMC2491705
- DOI: 10.1007/s00418-008-0452-5
The renal cortical interstitium: morphological and functional aspects
Abstract
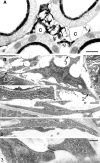
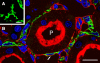
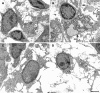
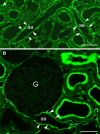
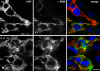
The renal interstitial compartment, situated between basement membranes of epithelia and vessels, contains two contiguous cellular networks. One network is formed by interstitial fibroblasts, the second one by dendritic cells. Both are in intimate contact with each other. Fibroblasts are interconnected by junctions and connected to basement membranes of vessels and tubules by focal adhesions. Fibroblasts constitute the "skeleton" of the kidney. In the renal cortex, fibroblasts produce erythropoietin and are distinguished from other interstitial cells by their prominent F-actin cytoskeleton, abundance of rough endoplasmic reticulum, and by ecto-5'-nucleotidase expression in their plasma membrane. The resident dendritic cells belong to the mononuclear phagocyte system and fulfil a sentinel function. They are characterized by their expression of MHC class II and CD11c. The central situation of fibroblasts suggests that signals from tubules, vessels, and inflammatory cells converge in fibroblasts and elicit an integrated response. Following tubular damage and inflammatory signals fibroblasts proliferate, change to the myofibroblast phenotype and increase their collagen production, potentially resulting in renal fibrosis. The acquisition of a profibrotic phenotype by fibroblasts in renal diseases is generally considered a main causal event in the progression of chronic renal failure. However, it might also be seen as a repair process.
Figures












References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1007/s004670050624', 'is_inner': False, 'url': 'https://doi.org/10.1007/s004670050624'}, {'type': 'PubMed', 'value': '10454789', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/10454789/'}]}
- Alcorn D, Maric C, McCausland J (1999) Development of the renal interstitium. Pediatr Nephrol 13:347–354 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1038/ki.1997.66', 'is_inner': False, 'url': 'https://doi.org/10.1038/ki.1997.66'}, {'type': 'PubMed', 'value': '9027726', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/9027726/'}]}
- Bachmann S, Ramasubbu K (1997) Immunohistochemical colocalization of the alpha-subunit of neutrophil NADPH oxidase and ecto-5′-nucleotidase in kidney and liver. Kidney Int 51:479–482 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '8429197', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/8429197/'}]}
- Bachmann S, Le Hir M, Eckardt KU (1993) Co-localization of erythropoietin mRNA and ecto-5′-nucleotidase immunoreactivity in peritubular cells of rat renal cortex indicates that fibroblasts produce erythropoietin. J Histochem Cytochem 41:335–341 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1159/000057607', 'is_inner': False, 'url': 'https://doi.org/10.1159/000057607'}, {'type': 'PubMed', 'value': '12021522', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12021522/'}]}
- Bertani T, Mazzucco G, Monga G (2002) How glomerular extracapillary proliferation might lead to loss of renal function: light microscopic and immunohistochemical investigation. Nephron 91:74–78 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1681/ASN.2005070730', 'is_inner': False, 'url': 'https://doi.org/10.1681/asn.2005070730'}, {'type': 'PubMed', 'value': '17135399', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/17135399/'}]}
- Broekema M, Harmsen MC, van Luyn MJ, Koerts JA, Petersen AH, van Kooten TG, van Goor H, Navis G, Popa ER (2007) Bone marrow-derived myofibroblasts contribute to the renal interstitial myofibroblast population and produce procollagen I after ischemia/reperfusion in rats. J Am Soc Nephrol 18:165–175 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

