Elucidating bone marrow edema and myelopoiesis in murine arthritis using contrast-enhanced magnetic resonance imaging
- PMID: 18576355
- PMCID: PMC2572869
- DOI: 10.1002/art.23546
Elucidating bone marrow edema and myelopoiesis in murine arthritis using contrast-enhanced magnetic resonance imaging
Abstract
Objective: While bone marrow edema (BME) detected by magnetic resonance imaging (MRI) is a biomarker of arthritis, its nature remains poorly understood due to the limitations of clinical studies. In this study, MRI of murine arthritis was used to elucidate its cellular composition and vascular involvement.
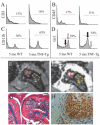
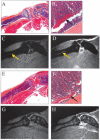
Methods: BME was quantified using normalized bone marrow intensity (NBMI) from precontrast MRI and normalized marrow contrast enhancement (NMCE) following intravenous administration of gadopentate dimeglumine. Wild-type (WT) and tumor necrosis factor (TNF)-transgenic mice were scanned from 2 to 5 months of age, followed by histologic or fluorescence-activated cell sorting (FACS) analysis of marrow. In efficacy studies, TNF-transgenic mice were treated with anti-TNF or placebo for 8 weeks, and then were studied using bimonthly MRI and histologic analysis.
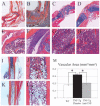
Results: NBMI values were similar in WT and TNF-transgenic mice at 2 months. The values in WT mice steadily decreased thereafter, with mean values becoming significantly different from those of TNF-transgenic mice at 3.5 months (mean +/- SD 0.29 +/- 0.08 versus 0.46 +/- 0.13; P < 0.05). Red to yellow marrow transformation occurred in WT but not TNF-transgenic mice, as observed histologically at 5 months. The marrow of TNF-transgenic mice that received anti-TNF therapy converted to yellow marrow, with lower NBMI values versus placebo at 6 weeks (mean +/- SD 0.26 +/- 0.07 versus 0.61 +/- 0.22; P < 0.05). FACS analysis of bone marrow revealed a significant correlation between NBMI values and CD11b+ monocytes (R2 = 0.91, P = 0.0028). Thresholds for "normal" red marrow versus pathologic BME were established, and it was also found that inflammatory marrow is highly permeable to contrast agent.
Conclusion: BME signals in TNF-transgenic mice are caused by yellow to red marrow conversion, with increased myelopoiesis and increased marrow permeability. The factors that mediate these changes warrant further investigation.
Figures



References
-
- Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–10. review. - PubMed
-
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61. review. - PubMed
-
- Feldmann M, Maini RN. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat Med. 2003;9:1245–50. published erratum appears in Nat Med 2003;9:1433. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR046545/AR/NIAMS NIH HHS/United States
- R01 AR051469/AR/NIAMS NIH HHS/United States
- R01 AR048697/AR/NIAMS NIH HHS/United States
- R21 DE017096/DE/NIDCR NIH HHS/United States
- AR-54041/AR/NIAMS NIH HHS/United States
- DE-17096/DE/NIDCR NIH HHS/United States
- P50 AR054041/AR/NIAMS NIH HHS/United States
- AR-43510/AR/NIAMS NIH HHS/United States
- R01 DK076876/DK/NIDDK NIH HHS/United States
- DK-64381/DK/NIDDK NIH HHS/United States
- AR-48697/AR/NIAMS NIH HHS/United States
- AR-51469/AR/NIAMS NIH HHS/United States
- R01 AR043510/AR/NIAMS NIH HHS/United States
- AR-46545/AR/NIAMS NIH HHS/United States
- K08 DK064381/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

