Toxicology study of repeat intracerebral administration of a measles virus derivative producing carcinoembryonic antigen in rhesus macaques in support of a phase I/II clinical trial for patients with recurrent gliomas
- PMID: 18576918
- PMCID: PMC2748764
- DOI: 10.1089/hum.2008.035
Toxicology study of repeat intracerebral administration of a measles virus derivative producing carcinoembryonic antigen in rhesus macaques in support of a phase I/II clinical trial for patients with recurrent gliomas
Abstract
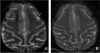
Gliomas have a dismal prognosis, with the median survival of patients with the most common histology, glioblastoma multiforme, being only 12-15 months. Development of novel therapeutic agents is urgently needed. We have previously demonstrated that oncolytic measles virus strains derived from the Edmonston vaccine lineage have significant antitumor activity against gliomas [Phuong, L.K., Allen, C., Peng, K.W., Giannini, C., Greiner, S., Teneyck, C.J., Mishra, P.K., Macura, S.I., Russell, S.J., Galanis, E.C. (2003). Cancer. Res. 63, 2462-2469]. MV-CEA is an Edmonston vaccine lineage measles virus strain engineered to express the marker peptide carcinoembryonic antigen (CEA): CEA levels can serve as a correlate of viral gene expression. In support of a phase I clinical trial of intratumoral and resection cavity administration of MV-CEA to patients with recurrent gliomas, we assessed the neurotoxicity of MV-CEA in adult immune male rhesus macaques (Macaca mulatta). The animals ' immune status and administration schedule mimicked the trial population and proposed administration schema. Macaca mulatta represents the prototype animal species for assessment of measles neurotoxicity. The animals were stereotactically administered either vehicle (n = 1) or MV-CEA at 2 x 10(5)or 2 x 10(6) TCID(50) (each, n = 2) in the right frontal lobe in two injections on days 1 and 5. Macaques were closely monitored clinically for neurotoxicity. Body weight, temperature, complete blood count, CEA, clinical chemistries, coagulation, complement levels, immunoglobulin, measles antibody titers, viremia, and shedding (buccal swabs) were tested at multiple time points. Furthermore, cisterna magna spinal taps were performed on day 9 and 1 year after the first viral dose administration, and samples were analyzed for protein, glucose, cell differential, and presence of MV-CEA. Magnetic resonance imaging (MRI) was performed between 4 and 5 months after article administration to assess for subclinical neurotoxicity. To date, 36+ months from study initiation there has been no clinical or biochemical evidence of toxicity, including lack of neurological symptoms, fever, or other systemic symptoms and lack of immunosuppression. Quantitative RT-PCR analysis of blood, buccal swabs, and cerebrospinal fluid (CSF) was negative for MV-CEA at all time points, with the exception of viral genome deletion in the blood of one asymptomatic animal at the 2 x 10(6) TCID(50) dose level on day 85. Vero cell overlays of CSF cells and supernatant were negative for viral recovery. There was no detection of CEA in serum or CSF at any time point. MRI scans were negative for imaging abnormalities and showed no evidence of encephalitis. Our results support the safety of CNS administration of MV-CEA in glioma patients. A clinical trial of intratumoral and resection cavity administration of MV-CEA in patients with recurrent glioblastoma multiforme is currently ongoing.
Figures


References
-
- Albrecht P. Shabo A.L. Burns G.R. Tauraso N.M. Experimental measles encephalitis in normal and cyclophosphamide-treated rhesus monkeys. J. Infect. Dis. 1972;126:154–161. - PubMed
-
- Allen C. Vongpunsawad S. Nakamura T. James C.D. Schroeder M. Cattaneo R. Giannini C. Krempski J. Peng K.W. Goble J.M. Uhm J.H. Russell S.J. Galanis E. Retargeted oncolytic measles strains entering via the EGFRvIII receptor maintain significant antitumor activity against gliomas with increased tumor specificity. Cancer Res. 2006;66:11840–11850. - PubMed
-
- Billeter M.A. Cattaneo R. Spielhofer P. Generation and properties of measles virus mutations typically associated with subacute sclerosing panencephalitis. In: Björnsson J., editor; Carp R.I., editor; Love A., editor; Wisniewski H.M., editor. Slow Infections of the Central Nervous System: The Legacy of Dr. Björn Sigurdsson. New York Academy of Sciences; New York: 1994. pp. 397–375. - PubMed
-
- Blechacz B. Splinter P.L. Greiner S. Myers R. Peng K.W. Federspiel M.J. Russell S.J. Larusso N.F. Engineered measles virus as a novel oncolytic viral therapy system for hepatocellular carcinoma. Hepatology. 2006;44:1465–1477. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

