HIV-1 Vif, APOBEC, and intrinsic immunity
- PMID: 18577210
- PMCID: PMC2443170
- DOI: 10.1186/1742-4690-5-51
HIV-1 Vif, APOBEC, and intrinsic immunity
Abstract
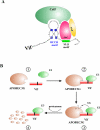
Members of the APOBEC family of cellular cytidine deaminases represent a recently identified group of proteins that provide immunity to infection by retroviruses and protect the cell from endogenous mobile retroelements. Yet, HIV-1 is largely immune to the intrinsic antiviral effects of APOBEC proteins because it encodes Vif (viral infectivity factor), an accessory protein that is critical for in vivo replication of HIV-1. In the absence of Vif, APOBEC proteins are encapsidated by budding virus particles and either cause extensive cytidine to uridine editing of negative sense single-stranded DNA during reverse transcription or restrict virus replication through deaminase-independent mechanisms. Thus, the primary function of Vif is to prevent encapsidation of APOBEC proteins into viral particles. This is in part accomplished by the ability of Vif to induce the ubiquitin-dependent degradation of some of the APOBEC proteins. However, Vif is also able to prevent encapsidation of APOBEC3G and APOBEC3F through degradation-independent mechanism(s). The goal of this review is to recapitulate current knowledge of the functional interaction of HIV-1 and its Vif protein with the APOBEC3 subfamily of proteins and to summarize our present understanding of the mechanism of APOBEC3-dependent retrovirus restriction.
Figures


Comment in
-
APOBEC3G encapsidation into HIV-1 virions: which RNA is it?Retrovirology. 2008 Jul 2;5:55. doi: 10.1186/1742-4690-5-55. Retrovirology. 2008. PMID: 18597677 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

