Low-level expression of HER2 and CK19 in normal peripheral blood mononuclear cells: relevance for detection of circulating tumor cells
- PMID: 18577250
- PMCID: PMC2423476
- DOI: 10.1186/1756-8722-1-2
Low-level expression of HER2 and CK19 in normal peripheral blood mononuclear cells: relevance for detection of circulating tumor cells
Abstract
Background: Detection of circulating tumor cells (CTC) in the blood of cancer patients may have prognostic and predictive significance. However, background expression of 'tumor specific markers' in peripheral blood mononuclear cells (PBMC) may confound these studies. The goal of this study was to identify the origin of Cytokeratin 19 (CK19) and HER-2 signal in PBMC and suggest an approach to enhance techniques involved in detection of CTC in breast cancer patients.
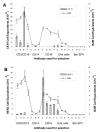
Methods: PBMC from healthy donors were isolated and fractionated into monocytes, lymphocytes, natural killer cells/granulocytes and epithelial populations using immunomagnetic selection and fluorescent cell-sorting for each cell type. RNA isolated from each fraction was analyzed for CK19, HER2 and Beta 2 microglobulin (B2M) using real-time qRT-PCR. Positive selection for epithelial cells and negative selection for NK/granulocytes were used in an attempt to reduce background expression of CK19 and HER2 markers.
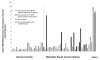
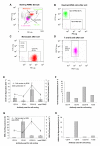
Results: In normal PBMC, CK19 was expressed in the lymphocyte population while HER-2 expression was highest in the NK/granulocyte population. Immunomagnetic selection for epithelial cells reduced background CK19 signal to a frequency of <5% in normal donors. Using negative selection, the majority (74-98%) of HER2 signal could be removed from PBMC. Positive selection methods are variably effective at reducing these background signals.
Conclusion: We present a novel method to improve the specificity of the traditional method of detecting CTC by identifying the source of the background signals and reducing them by negative immunoselection. Further studies are warranted to improve sensitivity and specificity of methods of detecting CTC will prove to be useful tools for clinicians in determining prognosis and monitoring treatment responses of breast cancer patients.
Figures
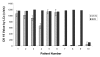
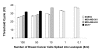
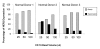
Similar articles
-
Circulating tumor cells in HER-2 positive metastatic breast cancer patients treated with trastuzumab and chemotherapy.Int J Biol Markers. 2009 Jan-Mar;24(1):1-10. doi: 10.1177/172460080902400101. Int J Biol Markers. 2009. PMID: 19404916
-
CK19, CK20, EGFR and HER2 status of circulating tumor cells in patients with breast cancer.Tumori. 2012 Mar-Apr;98(2):243-51. doi: 10.1177/030089161209800211. Tumori. 2012. PMID: 22677992
-
Detection of tumor cell-specific mRNA in the peripheral blood of patients with breast cancer—evaluation of several markers with real-time reverse transcription-PCR.Int J Mol Sci. 2013 Jan 8;14(1):1093-104. doi: 10.3390/ijms14011093. Int J Mol Sci. 2013. PMID: 23299436 Free PMC article.
-
Effects of Herceptin on circulating tumor cells in HER2 positive early breast cancer.Genet Mol Res. 2015 Mar 20;14(1):2099-103. doi: 10.4238/2015.March.20.20. Genet Mol Res. 2015. PMID: 25867356
-
Cytokeratin 19-positive circulating tumor cells in early breast cancer prognosis.Future Oncol. 2010 Feb;6(2):209-19. doi: 10.2217/fon.09.147. Future Oncol. 2010. PMID: 20146580 Review.
Cited by
-
HER-2 status of circulating tumor cells in a metastatic breast cancer cohort: A comparative study on characterization techniques.PLoS One. 2019 Sep 4;14(9):e0220906. doi: 10.1371/journal.pone.0220906. eCollection 2019. PLoS One. 2019. PMID: 31483799 Free PMC article.
-
Quantitative real-time RT-PCR and chromogenic in situ hybridization: precise methods to detect HER-2 status in breast carcinoma.BMC Cancer. 2009 Mar 23;9:90. doi: 10.1186/1471-2407-9-90. BMC Cancer. 2009. PMID: 19309522 Free PMC article.
-
Circulating tumor cells: clinically relevant molecular access based on a novel CTC flow cell.PLoS One. 2014 Jan 29;9(1):e86717. doi: 10.1371/journal.pone.0086717. eCollection 2014. PLoS One. 2014. PMID: 24489774 Free PMC article.
-
Target Antigen Attributes and Their Contributions to Clinically Approved Antibody-Drug Conjugates (ADCs) in Haematopoietic and Solid Cancers.Cancers (Basel). 2023 Mar 19;15(6):1845. doi: 10.3390/cancers15061845. Cancers (Basel). 2023. PMID: 36980732 Free PMC article. Review.
-
Fluorescence Analysis of Vitamin D Receptor Status of Circulating Tumor Cells (CTCS) in Breast Cancer: From Cell Models to Metastatic Patients.Int J Mol Sci. 2017 Jun 20;18(6):1318. doi: 10.3390/ijms18061318. Int J Mol Sci. 2017. PMID: 28632174 Free PMC article.
References
-
- Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, Schlimok G, Diel IJ, Gerber B, Gebauer G, Pierga JY, Marth C, Oruzio D, Wiedswang G, Solomayer EF, Kundt G, Strobl B, Fehm T, Wong GY, Bliss J, Vincent-Salomon A, Pantel K. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353:793–802. doi: 10.1056/NEJMoa050434. - DOI - PubMed
-
- Cote RJ, Rosen PP, Lesser ML, Old LJ, Osborne MP. Prediction of early relapse in patients with operable breast cancer by detection of occult bone marrow micrometastases. J Clin Oncol. 1991;9:1749–1756. - PubMed
-
- Berger U, Bettelheim R, Mansi JL, Easton D, Coombes RC, Neville AM. The relationship between micrometastases in the bone marrow, histopathologic features of the primary tumor in breast cancer and prognosis. Am J Clin Pathol. 1988;90:1–6. - PubMed
-
- Bostick PJ, Chatterjee S, Chi DD, Huynh KT, Giuliano AE, Cote R, Hoon DS. Limitations of specific reverse-transcriptase polymerase chain reaction markers in the detection of metastases in the lymph nodes and blood of breast cancer patients. J Clin Oncol. 1998;16:2632–2640. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

