The ubiquitin-protein ligase Nedd4-2 differentially interacts with and regulates members of the Tweety family of chloride ion channels
- PMID: 18577513
- PMCID: PMC3259790
- DOI: 10.1074/jbc.M803361200
The ubiquitin-protein ligase Nedd4-2 differentially interacts with and regulates members of the Tweety family of chloride ion channels
Abstract
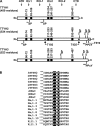
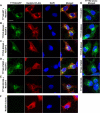
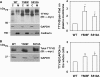
The Tweety proteins comprise a family of chloride ion channels with three members identified in humans (TTYH1-3) and orthologues in fly and murine species. In humans, increased TTYH2 expression is associated with cancer progression, whereas fly Tweety is associated with developmental processes. Structurally, Tweety proteins are characterized by five membrane-spanning domains and N-glycan modifications important for trafficking to the plasma membrane, where these proteins are oriented with the amino terminus located extracellularly and the carboxyl terminus cytoplasmically. In addition to N-glycosylation, ubiquitination mediated by the HECT type E3 ubiquitin ligase Nedd4-2 is a post-translation modification important in regulating membrane proteins. In the present study, we performed a comprehensive analysis of the ability of each of TTYH1-3 to interact with Nedd4-2 and to be ubiquitinated and regulated by this ligase. Our data indicate that Nedd4-2 binds to two family members, TTYH2 and TTYH3, which contain consensus PY ((L/P)PXY) binding sites for HECT type E3 ubiquitin ligases, but not to TTYH1, which lacks this motif. Consistently, Nedd4-2 ubiquitinates both TTYH2 and TTYH3. Importantly, we have shown that endogenous TTYH2 and Nedd4-2 are binding partners and demonstrated that the TTYH2 PY motif is essential for these interactions. We have also shown that Nedd4-2-mediated ubiquitination of TTYH2 is a critical regulator of cell surface and total cellular levels of this protein. These data, indicating that Nedd4-2 differentially interacts with and regulates TTYH1-3, will be important for understanding mechanisms controlling Tweety proteins in physiology and disease.
Figures





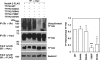
Similar articles
-
N-glycosylation analysis of the human Tweety family of putative chloride ion channels supports a penta-spanning membrane arrangement: impact of N-glycosylation on cellular processing of Tweety homologue 2 (TTYH2).Biochem J. 2008 May 15;412(1):45-55. doi: 10.1042/BJ20071722. Biochem J. 2008. PMID: 18260827
-
Nedd4-2 catalyzes ubiquitination and degradation of cell surface ENaC.J Biol Chem. 2007 Jul 13;282(28):20207-12. doi: 10.1074/jbc.M611329200. Epub 2007 May 14. J Biol Chem. 2007. PMID: 17502380
-
Regulation of the divalent metal ion transporter DMT1 and iron homeostasis by a ubiquitin-dependent mechanism involving Ndfips and WWP2.Blood. 2008 Nov 15;112(10):4268-75. doi: 10.1182/blood-2008-04-150953. Epub 2008 Sep 5. Blood. 2008. PMID: 18776082
-
The role of Nedd4/Nedd4-like dependant ubiquitylation in epithelial transport processes.Pflugers Arch. 2003 Jun;446(3):334-8. doi: 10.1007/s00424-003-1027-x. Epub 2003 Apr 16. Pflugers Arch. 2003. PMID: 12698368 Review.
-
Physiological Functions of the Ubiquitin Ligases Nedd4-1 and Nedd4-2.Physiology (Bethesda). 2024 Jan 1;39(1):18-29. doi: 10.1152/physiol.00023.2023. Epub 2023 Nov 14. Physiology (Bethesda). 2024. PMID: 37962894 Review.
Cited by
-
A genome-wide association study of caffeine-related sleep disturbance: confirmation of a role for a common variant in the adenosine receptor.Sleep. 2012 Jul 1;35(7):967-75. doi: 10.5665/sleep.1962. Sleep. 2012. PMID: 22754043 Free PMC article.
-
Progressive myoclonic epilepsy-associated gene KCTD7 is a regulator of potassium conductance in neurons.Mol Neurobiol. 2011 Aug;44(1):111-21. doi: 10.1007/s12035-011-8194-0. Epub 2011 Jun 28. Mol Neurobiol. 2011. PMID: 21710140
-
TTYH family members form tetrameric complexes at the cell membrane.Commun Biol. 2022 Aug 30;5(1):886. doi: 10.1038/s42003-022-03862-3. Commun Biol. 2022. PMID: 36042377 Free PMC article.
-
NEDD4-2 and the CLC-2 channel regulate neuronal excitability in the pathogenesis of mesial temporal lobe epilepsy.Sci Rep. 2024 Feb 28;14(1):4835. doi: 10.1038/s41598-024-52399-4. Sci Rep. 2024. PMID: 38418461 Free PMC article.
-
USP9X Deubiquitylates DVL2 to Regulate WNT Pathway Specification.Cell Rep. 2019 Jul 23;28(4):1074-1089.e5. doi: 10.1016/j.celrep.2019.06.083. Cell Rep. 2019. PMID: 31340145 Free PMC article.
References
-
- Sullivan, M., and Morgan, D. O. (2007) Nat. Rev. Mol. Cell Biol. 8894 -903 - PubMed
-
- Muratani, M., and Tansey, W. P. (2003) Nat. Rev. Mol. Cell Biol. 4192 -201 - PubMed
-
- Liu, Y. C. (2004) Annu. Rev. Immunol. 2281 -127 - PubMed
-
- DiAntonio, A., and Hicke, L. (2004) Annu. Rev. Neurosci. 27223 -246 - PubMed
-
- Mukhopadhyay, D., and Riezman, H. (2007) Science 315201 -205 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

