Paroxysmal exercise-induced dyskinesia and epilepsy is due to mutations in SLC2A1, encoding the glucose transporter GLUT1
- PMID: 18577546
- PMCID: PMC2442425
- DOI: 10.1093/brain/awn113
Paroxysmal exercise-induced dyskinesia and epilepsy is due to mutations in SLC2A1, encoding the glucose transporter GLUT1
Abstract
Paroxysmal exercise-induced dyskinesia (PED) can occur in isolation or in association with epilepsy, but the genetic causes and pathophysiological mechanisms are still poorly understood. We performed a clinical evaluation and genetic analysis in a five-generation family with co-occurrence of PED and epilepsy (n = 39), suggesting that this combination represents a clinical entity. Based on a whole genome linkage analysis we screened SLC2A1, encoding the glucose transporter of the blood-brain-barrier, GLUT1 and identified heterozygous missense and frameshift mutations segregating in this and three other nuclear families with a similar phenotype. PED was characterized by choreoathetosis, dystonia or both, affecting mainly the legs. Predominant epileptic seizure types were primary generalized. A median CSF/blood glucose ratio of 0.52 (normal >0.60) in the patients and a reduced glucose uptake by mutated transporters compared with the wild-type as determined in Xenopus oocytes confirmed a pathogenic role of these mutations. Functional imaging studies implicated alterations in glucose metabolism in the corticostriate pathways in the pathophysiology of PED and in the frontal lobe cortex in the pathophysiology of epileptic seizures. Three patients were successfully treated with a ketogenic diet. In conclusion, co-occurring PED and epilepsy can be due to autosomal dominant heterozygous SLC2A1 mutations, expanding the phenotypic spectrum associated with GLUT1 deficiency and providing a potential new treatment option for this clinical syndrome.
Figures




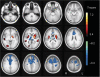

References
-
- Auburger G, Ratzlaff T, Lunkes A, Nelles HW, Leube B, Binkofski F, et al. A gene for autosomal dominant paroxysmal choreoathetosis/spasticity (CSE) maps to the vicinity of a potassium channel gene cluster on chromosome 1p, probably within 2 cM between D1S443 and D1S197. Genomics. 1996;31:90–4. - PubMed
-
- Berkovic SF. Paroxysmal movement disorders and epilepsy: links across the channel. Neurology. 2000;55:169–70. - PubMed
-
- Bhatia KP, Soland VL, Bhatt MH, Quinn NP, Marsden CD. Paroxysmal exercise-induced dystonia: eight new sporadic cases and a review of the literature. Mov Disord. 1997;12:1007–12. - PubMed
-
- Bing F, Dananchet Y, Vercueil L. [A family with exercise-induced paroxysmal dystonia and childhood absence epilepsy] Rev Neurol (Paris) 2005;161:817–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

