Caveolin-1-dependent beta1 integrin endocytosis is a critical regulator of fibronectin turnover
- PMID: 18577581
- PMCID: PMC2587120
- DOI: 10.1242/jcs.014977
Caveolin-1-dependent beta1 integrin endocytosis is a critical regulator of fibronectin turnover
Abstract
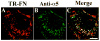
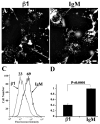
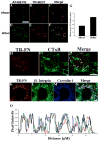
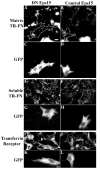
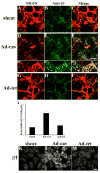
beta1 integrins are major cell surface receptors for fibronectin. Some integrins, including beta1 integrins, are known to undergo constitutive endocytosis and recycling. Integrin endocytosis/recycling has been implicated in the regulation of cell migration. However, the mechanisms by which integrin endocytosis/recycling regulates cell migration, and other biological consequences of integrin trafficking are not completely understood. We previously showed that turnover of extracellular matrix (ECM) fibronectin occurs via receptor-mediated endocytosis. Here, we investigate the biological relevance of beta1 integrin endocytosis to fibronectin matrix turnover. First, we demonstrate that beta1 integrins, including alpha5beta1 play an important role in endocytosis and turnover of matrix fibronectin. Second, we show that caveolin-1 constitutively regulates endocytosis of alpha5beta1 integrins, and that alpha5beta1 integrin endocytosis can occur in the absence of fibronectin and fibronectin matrix. We also show that downregulation of caveolin-1 expression by siRNA results in marked reduction of beta1 integrin and fibronectin endocytosis. Hence, caveolin-1-dependent beta1 integrin and fibronectin endocytosis plays a critical role in fibronectin matrix turnover, and may contribute to abnormal ECM remodeling that occurs in fibrotic disorders.
Figures


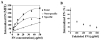




References
-
- Aplin AE, Howe AK, Juliano RL. Cell adhesion molecules, signal transduction and cell growth. Current Opinion Cell Biol. 1999;11:737–744. - PubMed
-
- Bazzoni G, Shih DT, Buck CA, Hemler ME. Monoclonal antibody 9EG7 defines a novel beta 1 integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J Biol Chem. 1995;270:25570–7. - PubMed
-
- Beardsley A, Fang K, Mertz H, Castranova V, Friend S, Liu J. Loss of caveolin-1 polarity impedes endothelial cell polarization and directional movement. J Biol Chem. 2005;280:3541–7. - PubMed
-
- Benmerah A, Bayrou M, Cerf-Bensussan N, Dautry-Varsat A. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J Cell Sci. 1999;112:1303–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

