Spermidine exodus and oxidation in the apoplast induced by abiotic stress is responsible for H2O2 signatures that direct tolerance responses in tobacco
- PMID: 18577660
- PMCID: PMC2483379
- DOI: 10.1105/tpc.108.059733
Spermidine exodus and oxidation in the apoplast induced by abiotic stress is responsible for H2O2 signatures that direct tolerance responses in tobacco
Abstract
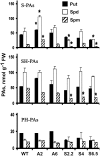
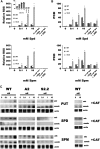
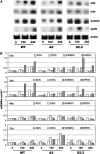
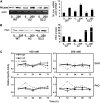
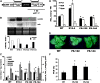
Polyamines (PAs) exert a protective effect against stress challenges, but their molecular role in this remains speculative. In order to detect the signaling role of apoplastic PA-derived hydrogen peroxide (H2O2) under abiotic stress, we developed a series of tobacco (Nicotiana tabacum cv Xanthi) transgenic plants overexpressing or downregulating apoplastic polyamine oxidase (PAO; S-pao and A-pao plants, respectively) or downregulating S-adenosyl-l-methionine decarboxylase (samdc plants). Upon salt stress, plants secreted spermidine (Spd) into the apoplast, where it was oxidized by the apoplastic PAO, generating H2O2. A-pao plants accumulated less H2O2 and exhibited less programmed cell death (PCD) than did wild-type plants, in contrast with S-pao and samdc downregulating plants. Induction of either stress-responsive genes or PCD was dependent on the level of Spd-derived apoplastic H2O2. Thus, in wild-type and A-pao plants, stress-responsive genes were efficiently induced, although in the latter at a lower rate, while S-pao plants, with higher H2O2 levels, failed to accumulate stress-responsive mRNAs, inducing PCD instead. Furthermore, decreasing intracellular PAs, while keeping normal apoplastic Spd oxidation, as in samdc downregulating transgenic plants, caused enhanced salinity-induced PCD. These results reveal that salinity induces the exodus of Spd into the apoplast, where it is catabolized by PAO, producing H2O2. The accumulated H2O2 results in the induction of either tolerance responses or PCD, depending also on the levels of intracellular PAs.
Figures
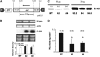





References
-
- Agalou, A., et al. (2008). A genome-wide survey of HD-Zip genes in rice and analysis of drought-responsive family members. Plant Mol. Biol. 66 87–103. - PubMed
-
- Alcazar, R., Marco, F., Cuevas, J.C., Patron, M., Ferrando, A., Carrasco, P., Tiburcio, A.F., and Altabella, T. (2006). Involvement of polyamines in plant response to abiotic stress. Biotechnol. Lett. 28 1867–1876. - PubMed
-
- Angelini, R., Federico, R., and Bonfante, P. (1995). Maize polyamine oxidase: Antibody production and ultrastructural localization. J. Plant Physiol. 145 686–692.
-
- Bouchereau, A., Aziz, A., Larher, F., and Martin-Tanguy, J. (1999). Polyamines and environmental challenges: Recent development. Plant Sci. 140 103–125.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

