The familial Mediterranean fever protein, pyrin, is cleaved by caspase-1 and activates NF-kappaB through its N-terminal fragment
- PMID: 18577712
- PMCID: PMC2518886
- DOI: 10.1182/blood-2008-01-134932
The familial Mediterranean fever protein, pyrin, is cleaved by caspase-1 and activates NF-kappaB through its N-terminal fragment
Abstract
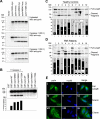
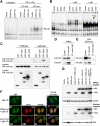
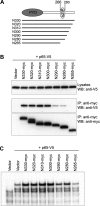
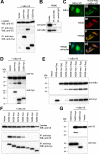
Familial Mediterranean fever (FMF) is an autoinflammatory disease caused by mutations in MEFV, which encodes a 781-amino acid protein denoted pyrin. We have previously shown that pyrin regulates caspase-1 activation and IL-1beta production through interaction of its N-terminal PYD motif with the ASC adapter protein, and also modulates IL-1beta production by interaction of its C-terminal B30.2 domain with the catalytic domains of caspase-1. We now asked whether pyrin might itself be a caspase-1 substrate, and found that pyrin is cleaved by caspase-1 at Asp330, a site remote from the B30.2 domain. Pyrin variants harboring FMF-associated B30.2 mutations were cleaved more efficiently than wild-type pyrin. The N-terminal cleaved fragment interacted with the p65 subunit of NF-kappaB and with IkappaB-alpha through its 15-aa bZIP basic domain and adjacent sequences, respectively, and translocated to the nucleus. The interaction of the N-terminal fragment with p65 enhanced entrance of p65 into the nucleus. The interaction of N-terminal pyrin with IkappaB-alpha induced calpain-mediated degradation of IkappaB-alpha, thus potentiating NF-kappaB activation. Absolute and relative quantities of cleaved pyrin and IkappaB-alpha degradation products were substantially increased in leukocytes from FMF patients compared with healthy controls. Our data support a new pyrin/caspase-1 pathway for NF-kappaB activation.
Figures


References
-
- Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever: The International FMF Consortium. Cell. 1997;90:797–807. - PubMed
-
- A candidate gene for familial Mediterranean fever: The French FMF Consortium. Nat Genet. 1997;17:25–31. - PubMed
-
- Centola M, Wood G, Frucht DM, et al. The gene for familial Mediterranean fever, MEFV, is expressed in early leukocyte development and is regulated in response to inflammatory mediators. Blood. 2000;95:3223–3231. - PubMed
-
- Diaz A, Hu C, Kastner DL, et al. Lipopolysaccharide-induced expression of multiple alternatively spliced MEFV transcripts in human synovial fibroblasts: a prominent splice isoform lacks the C-terminal domain that is highly mutated in familial Mediterranean fever. Arthritis Rheum. 2004;50:3679–3689. - PubMed
-
- Touitou I. The spectrum of Familial Mediterranean Fever (FMF) mutations. Eur J Hum Genet. 2001;9:473–483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

