Histoplasma capsulatum manifests preferential invasion of phagocytic subpopulations in murine lungs
- PMID: 18577715
- PMCID: PMC2516902
- DOI: 10.1189/jlb.0308154
Histoplasma capsulatum manifests preferential invasion of phagocytic subpopulations in murine lungs
Abstract
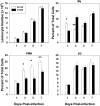
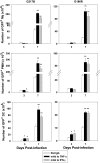
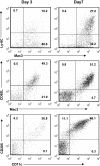
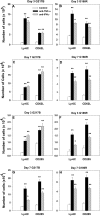
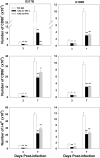
Numerous in vitro studies have demonstrated that Histoplasma capsulatum is engulfed by the diverse populations of phagocytic cells including monocytes/macrophages (Mphi), immature dendritic cells (DC), and neutrophils. The in vivo distribution of H. capsulatum has yet to be examined following an intrapulmonary challenge. To accomplish this goal, we engineered GFP into two genetically dissimilar strains of H. capsulatum, G217B and 186R. C57BL/6 mice were infected with each of these strains, and we analyzed the distribution of this fungus in the three major phagocytic populations on successive days. Yeast cells were found in all three populations of cells from Days 1 through 7. Proportionally, DC dominated at Day 1, whereas the majority of yeast cells was detected in neutrophils thereafter. Yeast cells were present in inflammatory and resident Mphi on Day 3, but on Day 7, they were chiefly in inflammatory Mphi. Yeast cells were predominantly in a CD11c(+intermediate/high), F4/80(-), CD11b(+), Ly-6C(+), CD205(+) DC population. Neutralization of TNF-alpha or IFN-gamma produced a significant redistribution of yeast cells. These results reveal the complex nature of intracellular residence of this fungus. Moreover, the findings demonstrate that there is a skewing in the subpopulations of cells that are infected, especially DC.
Figures



References
-
- Baughman R P, Kim C K, Vinegar A, Hendricks D E, Schmidt D J, Bullock W E. The pathogenesis of experimental pulmonary histoplasmosis. Correlative studies of histopathology, bronchoalveolar lavage, and respiratory function. Am Rev Respir Dis. 1986;134:771–776. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

