Inducible re-expression of p16 in an orthotopic mouse model of pancreatic cancer inhibits lymphangiogenesis and lymphatic metastasis
- PMID: 18577984
- PMCID: PMC2453030
- DOI: 10.1038/sj.bjc.6604457
Inducible re-expression of p16 in an orthotopic mouse model of pancreatic cancer inhibits lymphangiogenesis and lymphatic metastasis
Abstract
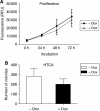
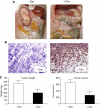
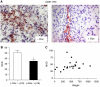
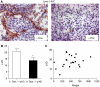
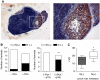
Functional inactivation of the tumour suppressor protein p16(INK4a) constitutes a key event in the multistep process of pancreatic ductal cell transformation. However, the significance of p16 inactivation for complex and tissue-specific aspects of pancreatic cancer progression, such as angiogenesis and metastasis, is less understood. Here, we inducibly re-expressed p16 in vivo in an orthotopic model of pancreatic cancer and examined the impact on these clinically relevant aspects of pancreatic cancer tumour biology. Consistent with previous work in subcutaneous xenograft models, we found p16 capable of reducing primary tumour growth. In addition, p16 restitution resulted in a marked reduction of tumour angiogenesis, largely accounted for by a p16-dependent inhibition of lymphangiogenesis. In excellent agreement with the antilymphangiogenic effect, re-expression of p16 almost completely prevented lymph node metastases of MiaPaca-2 pancreatic tumours. To our knowledge, this is the first report that experimentally links the tumour suppressor p16 to the process of lymphangiogenesis.
Figures

References
-
- Ahmed SA, Gogal Jr RM, Walsh JE (1994) A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J Immunol Methods 170: 211–224 - PubMed
-
- Alves F, Contag S, Missbach M, Kaspareit J, Nebendahl K, Borchers U, Heidrich B, Streich R, Hiddemann W (2001) An orthotopic model of ductal adenocarcinoma of the pancreas in severe combined immunodeficient mice representing all steps of the metastatic cascade. Pancreas 23: 227–235 - PubMed
-
- Bardeesy N, Aguirre AJ, Chu GC, Cheng KH, Lopez LV, Hezel AF, Feng B, Brennan C, Weissleder R, Mahmood U, Hanahan D, Redston MS, Chin L, DePinho RA (2006) Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc Natl Acad Sci USA 103: 5947–5952 - PMC - PubMed
-
- Blouw B, Song H, Tihan T, Bosze J, Ferrara N, Gerber HP, Johnson RS, Bergers G (2003) The hypoxic response of tumors is dependent on their microenvironment. Cancer Cell 4: 133–146 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

