Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer: results of secondary end points analyses
- PMID: 18577990
- PMCID: PMC2453014
- DOI: 10.1038/sj.bjc.6604436
Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer: results of secondary end points analyses
Abstract
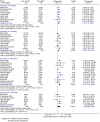
In advanced pancreatic cancer, level one evidence has established a significant survival advantage with chemotherapy, compared to best supportive care. The treatment-associated toxicity needs to be evaluated. This study examines the secondary outcome measures for chemotherapy in advanced pancreatic cancer using meta-analyses. A systematic review was undertaken employing Cochrane methodology, with search of databases, conference proceedings and trial registers. The secondary end points were progression-free survival (PFS)/time to progression (TTP) (summarised using the hazard ratio (HR)), response rate and toxicity (summarised using relative risk). There was no significant advantage of 5FU combinations vs 5FU alone for TTP (HR=1.02; 95% CI=0.85-1.23) and toxicity. Progression-free survival (HR 0.78; CI 0.70-0.88), TTP (HR=0.85; 95% CI=0.72-0.99) and overall response rate (RR=0.56; 95% CI=0.46-0.68) were significantly better for gemcitabine combination chemotherapy, but offset by the greater grade 3/4 toxicity thrombocytopenia (RR=1.94; 95% CI=1.32-2.84), leucopenia (RR=1.46; 95% CI=1.15-1.86), neutropenia (RR=1.48; 95% CI=1.07-2.05), nausea (RR=1.77; 95% CI=1.37-2.29), vomiting (RR=1.64; 95% CI=1.24-2.16) and diarrhoea (RR=2.73; 95% CI=1.87-3.98). There is no significant advantage on secondary end point analyses for administering 5FU in combination over 5FU alone. There is improved PFS/TTP and response rate, with gemcitabine-based combinations, although this comes with greater toxicity.
Figures




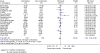

References
-
- Abou-Alfa GK, Letourneau R, Harker G, Modiano M, Hurwitz H, Tchekmedyian NS, Feit K, Ackerman J, De Jager RL, Eckhardt SG, O'Reilly EM (2006) Randomised phase III study of exatecan and gemcitabine compared with gemcitabine alone in untreated advanced pancreatic cancer. J Clin Oncol 24: 4441–4447 - PubMed
-
- Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Benson III AB (2002) Phase III study of gemcitabine in combination with fluorouracil vs gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol 20: 3270–3275 - PubMed
-
- Bria E, Milella M, Gelibter A, Cuppone F, Pino MS, Ruggeri EM, Carlini P, Nisticò C, Terzoli E, Cognetti F, Giannarelli D (2007) Gemcitabine-based combinations for inoperable pancreatic cancer: have we made real progress? A meta-analysis of 20 phase 3 trials. Cancer 110: 525–533 - PubMed
-
- Burris H, Moore M, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomised trial. J Clin Oncol 15: 2403–2413 - PubMed
-
- Cancer Research UK (2006) Cancer Stats Incidence, http://info.cancerresearchuk.org/cancerstats
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

