The B cell antigen receptor and overexpression of MYC can cooperate in the genesis of B cell lymphomas
- PMID: 18578569
- PMCID: PMC2435152
- DOI: 10.1371/journal.pbio.0060152
The B cell antigen receptor and overexpression of MYC can cooperate in the genesis of B cell lymphomas
Abstract
A variety of circumstantial evidence from humans has implicated the B cell antigen receptor (BCR) in the genesis of B cell lymphomas. We generated mouse models designed to test this possibility directly, and we found that both the constitutive and antigen-stimulated state of a clonal BCR affected the rate and outcome of lymphomagenesis initiated by the proto-oncogene MYC. The tumors that arose in the presence of constitutive BCR differed from those initiated by MYC alone and resembled chronic B cell lymphocytic leukemia/lymphoma (B-CLL), whereas those that arose in response to antigen stimulation resembled large B-cell lymphomas, particularly Burkitt lymphoma (BL). We linked the genesis of the BL-like tumors to antigen stimulus in three ways. First, in reconstruction experiments, stimulation of B cells by an autoantigen in the presence of overexpressed MYC gave rise to BL-like tumors that were, in turn, dependent on both MYC and the antigen for survival and proliferation. Second, genetic disruption of the pathway that mediates signaling from the BCR promptly killed cells of the BL-like tumors as well as the tumors resembling B-CLL. And third, growth of the murine BL could be inhibited by any of three distinctive immunosuppressants, in accord with the dependence of the tumors on antigen-induced signaling. Together, our results provide direct evidence that antigenic stimulation can participate in lymphomagenesis, point to a potential role for the constitutive BCR as well, and sustain the view that the constitutive BCR gives rise to signals different from those elicited by antigen. The mouse models described here should be useful in exploring further the pathogenesis of lymphomas, and in preclinical testing of new therapeutics.
Conflict of interest statement
Figures

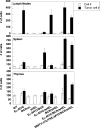



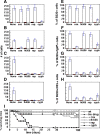
References
-
- Jaffe E, Harris NL, Stein H, Vardiman JW. World Health Organization classification of tumors. In: Kleihuis P, Sobin L, editors. Pathology and genetics of tumors of hematopoietic and lymphoid tissues. Lyon (France): Interantional Agency for Research on Cancer; 2001.
-
- Leoncini L, Delsol G, Gascoyne RD, Harris NL, Pileri SA, et al. Aggressive B-cell lymphomas: a review based on the workshop of the XI Meeting of the European Association for Haematopathology. Histopathology. 2005;46:241–255. - PubMed
-
- Küppers R. Mechanisms of B-cell lymphoma pathogenesis. Nat Rev Cancer. 2005;5:251–262. - PubMed
-
- Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–1083. - PubMed
-
- Neuberger MS. Antigen receptor signaling gives lymphocytes a long life. Cell. 1997;90:971–973. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

