Mechanisms of hemoglobin adaptation to high altitude hypoxia
- PMID: 18578646
- PMCID: PMC3140315
- DOI: 10.1089/ham.2007.1079
Mechanisms of hemoglobin adaptation to high altitude hypoxia
Abstract
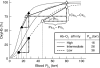
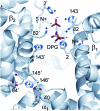
Evidence from a number of vertebrate taxa suggests that modifications of hemoglobin (Hb) function may often play a key role in mediating an adaptive response to high altitude hypoxia. The respiratory functions of Hb are a product of the protein's intrinsic O(2)-binding affinity and its interactions with allosteric effectors such as protons, chloride ions, CO(2), and organic phosphates. Here we review several case studies involving high altitude vertebrates where it has been possible to identify specific mechanisms of Hb adaptation to hypoxia. In addition to comparative studies of Hbs from diverse animal species, functional studies of human Hb mutants also suggest that there is ample scope for evolutionary adjustments in Hb-O(2) affinity through alterations of the equilibrium constants of O(2) binding to deoxy- and oxyHb or through changes in the allosteric equilibrium constants for the transition between the deoxy- and oxyHb quaternary structures. It may be the case that certain evolutionary paths are followed more often than others simply because they are subject to less stringent pleiotropic constraints.
Figures


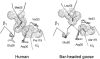

References
-
- Arnone A. Mechanism of action of hemoglobin. Ann. Rev. Med. 1974;25:123–130. - PubMed
-
- Baldwin J. Chothia C. Haemoglobin: the structural changes related to ligand binding and its allosteric mechanism. J. Mol. Biol. 1979;129:175–220. - PubMed
-
- Bettati S. Mozzarelli A. Perutz M.F. Allosteric mechanism of hemoglobin: rupture of salt bridges raises oxygen affinity of the T-structure. J. Mol. Biol. 1983;281:581–585. - PubMed
-
- Bencowitz H.Z. Wagner P.D. West J.B. Effect of change in P50 on exercise tolerance at high-altitude: a theoretical study. J. Appl. Physiol. 1982;53:1487–1495. - PubMed
-
- Bouverot P. Springer-Verlag; Berlin: 1985. Adaptation to Altitude-Hypoxia in Vertebrates.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

