Biochemical characterization and cellular imaging of a novel, membrane permeable fluorescent cAMP analog
- PMID: 18578870
- PMCID: PMC2443153
- DOI: 10.1186/1471-2091-9-18
Biochemical characterization and cellular imaging of a novel, membrane permeable fluorescent cAMP analog
Abstract
Background: A novel fluorescent cAMP analog (8-[Pharos-575]- adenosine-3', 5'-cyclic monophosphate) was characterized with respect to its spectral properties, its ability to bind to and activate three main isoenzymes of the cAMP-dependent protein kinase (PKA-Ialpha, PKA-IIalpha, PKA-IIbeta) in vitro, its stability towards phosphodiesterase and its ability to permeate into cultured eukaryotic cells using resonance energy transfer based indicators, and conventional fluorescence imaging.
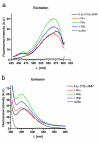
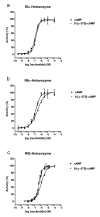
Results: The Pharos fluorophore is characterized by a Stokes shift of 42 nm with an absorption maximum at 575 nm and the emission peaking at 617 nm. The quantum yield is 30%. Incubation of the compound to RIIalpha and RIIbeta subunits increases the amplitude of excitation and absorption maxima significantly; no major change was observed with RIalpha. In vitro binding of the compound to RIalpha subunit and activation of the PKA-Ialpha holoenzyme was essentially equivalent to cAMP; RII subunits bound the fluorescent analog up to ten times less efficiently, resulting in about two times reduced apparent activation constants of the holoenzymes compared to cAMP. The cellular uptake of the fluorescent analog was investigated by cAMP indicators. It was estimated that about 7 muM of the fluorescent cAMP analog is available to the indicator after one hour of incubation and that about 600 muM of the compound had to be added to intact cells to half-maximally dissociate a PKA type IIalpha sensor.
Conclusion: The novel analog combines good membrane permeability- comparable to 8-Br-cAMP - with superior spectral properties of a modern, red-shifted fluorophore. GFP-tagged regulatory subunits of PKA and the analog co-localized. Furthermore, it is a potent, PDE-resistant activator of PKA-I and -II, suitable for in vitro applications and spatial distribution evaluations in living cells.
Figures





Similar articles
-
Novel, isotype-specific sensors for protein kinase A subunit interaction based on bioluminescence resonance energy transfer (BRET).Cell Signal. 2006 Oct;18(10):1616-25. doi: 10.1016/j.cellsig.2006.01.013. Epub 2006 Mar 9. Cell Signal. 2006. PMID: 16524697
-
Probing cAMP-dependent protein kinase holoenzyme complexes I alpha and II beta by FT-IR and chemical protein footprinting.Biochemistry. 2004 Feb 24;43(7):1908-20. doi: 10.1021/bi0354435. Biochemistry. 2004. PMID: 14967031
-
Differential effects of substrate on type I and type II PKA holoenzyme dissociation.Biochemistry. 2004 May 18;43(19):5629-36. doi: 10.1021/bi0499157. Biochemistry. 2004. PMID: 15134437
-
The ways in which hormones change cyclic adenosine 3',5'-monophosphate-dependent protein kinase subunits, and how such changes affect cell behavior.Endocr Rev. 1993 Oct;14(5):632-50. doi: 10.1210/edrv-14-5-632. Endocr Rev. 1993. PMID: 8262010 Review.
-
Protein kinase A: regulation and receptor-mediated delivery of antisense oligonucleotides and cytotoxic drugs.Ann N Y Acad Sci. 2002 Jun;968:158-72. doi: 10.1111/j.1749-6632.2002.tb04334.x. Ann N Y Acad Sci. 2002. PMID: 12119275 Review.
Cited by
-
Mechanisms of cAMP compartmentation in cardiac myocytes: experimental and computational approaches to understanding.J Physiol. 2021 Oct;599(20):4527-4544. doi: 10.1113/JP280801. Epub 2021 Sep 27. J Physiol. 2021. PMID: 34510451 Free PMC article. Review.
-
Stimulation of proglucagon gene expression by human GPR119 in enteroendocrine L-cell line GLUTag.Mol Endocrinol. 2013 Aug;27(8):1267-82. doi: 10.1210/me.2013-1029. Epub 2013 Jun 24. Mol Endocrinol. 2013. PMID: 23798572 Free PMC article.
-
Structure-guided design of selective Epac1 and Epac2 agonists.PLoS Biol. 2015 Jan 20;13(1):e1002038. doi: 10.1371/journal.pbio.1002038. eCollection 2015 Jan. PLoS Biol. 2015. PMID: 25603503 Free PMC article.
-
cAMP-dependent activation of protein kinase A as a therapeutic target of skin hyperpigmentation by diphenylmethylene hydrazinecarbothioamide.Br J Pharmacol. 2015 Jul;172(13):3434-45. doi: 10.1111/bph.13134. Epub 2015 Apr 24. Br J Pharmacol. 2015. PMID: 25766244 Free PMC article.
-
Fluorophore-Labeled Cyclic Nucleotides as Potent Agonists of Cyclic Nucleotide-Regulated Ion Channels.Chembiochem. 2020 Aug 17;21(16):2311-2320. doi: 10.1002/cbic.202000116. Epub 2020 May 4. Chembiochem. 2020. PMID: 32227403 Free PMC article.
References
-
- Cremo CR. Fluorescent nucleotides: synthesis and characterization. Methods Enzymol. 2003;360:128–177. - PubMed
-
- Builder SE, Beavo JA, Krebs EG. Stoichiometry of cAMP and 1,N6-etheno-cAMP binding to protein kinase. J Biol Chem. 1980;255:2350–2354. - PubMed
-
- Tsou KC, Yip KF, Lo KW. 1,N6-etheno-2-aza-adenosine 3',5'-monophosphate: a new fluorescent substrate for cycle nucleotide phosphodiesterase. Anal Biochem. 1974;60:163–169. - PubMed
-
- Scott SP, Tanaka JC. Molecular interactions of 3',5'-cyclic purine analogues with the binding site of retinal rod ion channels. Biochemistry. 1995;34:2338–2347. - PubMed
-
- Alfonso A, Estevez M, Louzao MC, Vieytes MR, Botana LM. Determination of phosphodiesterase activity in rat mast cells using the fluorescent cAMP analogue anthraniloyl cAMP. Cell Signal. 1995;7:513–518. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

