Mechanism of Ca2+ disruption in Alzheimer's disease by presenilin regulation of InsP3 receptor channel gating
- PMID: 18579078
- PMCID: PMC2495086
- DOI: 10.1016/j.neuron.2008.04.015
Mechanism of Ca2+ disruption in Alzheimer's disease by presenilin regulation of InsP3 receptor channel gating
Abstract
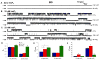
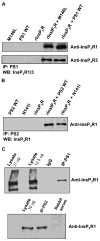
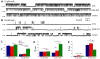
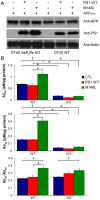
Mutations in presenilins (PS) are the major cause of familial Alzheimer's disease (FAD) and have been associated with calcium (Ca2+) signaling abnormalities. Here, we demonstrate that FAD mutant PS1 (M146L)and PS2 (N141I) interact with the inositol 1,4,5-trisphosphate receptor (InsP3R) Ca2+ release channel and exert profound stimulatory effects on its gating activity in response to saturating and suboptimal levels of InsP3. These interactions result in exaggerated cellular Ca2+ signaling in response to agonist stimulation as well as enhanced low-level Ca2+signaling in unstimulated cells. Parallel studies in InsP3R-expressing and -deficient cells revealed that enhanced Ca2+ release from the endoplasmic reticulum as a result of the specific interaction of PS1-M146L with the InsP3R stimulates amyloid beta processing,an important feature of AD pathology. These observations provide molecular insights into the "Ca2+ dysregulation" hypothesis of AD pathogenesis and suggest novel targets for therapeutic intervention.
Figures




References
-
- Balaban RS. Cardiac energy metabolism homeostasis: role of cytosolic calcium. J Mol Cell Cardiol. 2002;34:1259–1271. - PubMed
-
- Barrow PA, Empson RM, Gladwell SJ, Anderson CM, Killick R, Yu X, Jefferys JG, Duff K. Functional phenotype in transgenic mice expressing mutant human presenilin-1. Neurobiol Dis. 2000;7:119–126. - PubMed
-
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. - PubMed
-
- Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403. - PubMed
-
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

