Small heat shock proteins in smooth muscle
- PMID: 18579210
- PMCID: PMC2581864
- DOI: 10.1016/j.pharmthera.2008.04.005
Small heat shock proteins in smooth muscle
Abstract
The small heat shock proteins (HSPs) HSP20, HSP27 and alphaB-crystallin are chaperone proteins that are abundantly expressed in smooth muscles are important modulators of muscle contraction, cell migration and cell survival. This review focuses on factors regulating expression of small HSPs in smooth muscle, signaling pathways that regulate macromolecular structure and the biochemical and cellular functions of small HSPs. Cellular processes regulated by small HSPs include chaperoning denatured proteins, maintaining cellular redox state and modifying filamentous actin polymerization. These processes influence smooth muscle proliferation, cell migration, cell survival, muscle contraction and synthesis of signaling proteins. Understanding functions of small heat shock proteins is relevant to mechanisms of disease in which dysfunctional smooth muscle causes symptoms, or is a target of drug therapy. One example is that secreted HSP27 may be a useful marker of inflammation during atherogenesis. Another is that phosphorylated HSP20 which relaxes smooth muscle may prove to be highly relevant to treatment of hypertension, vasospasm, asthma, premature labor and overactive bladder. Because small HSPs also modulate smooth muscle proliferation and cell migration they may prove to be targets for developing effective, novel treatments of clinical problems arising from remodeling of smooth muscle in vascular, respiratory and urogenital systems.
Figures


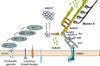
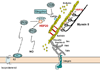
Similar articles
-
Role of the small heat shock proteins in regulating vascular smooth muscle tone.J Am Coll Surg. 2005 Jul;201(1):30-6. doi: 10.1016/j.jamcollsurg.2005.03.017. J Am Coll Surg. 2005. PMID: 15978441
-
Identification of peptides in human Hsp20 and Hsp27 that possess molecular chaperone and anti-apoptotic activities.Biochem J. 2015 Jan 1;465(1):115-25. doi: 10.1042/BJ20140837. Biochem J. 2015. PMID: 25332102 Free PMC article.
-
Phosphorylated HSP20 modulates the association of thin-filament binding proteins: caldesmon with tropomyosin in colonic smooth muscle.Am J Physiol Gastrointest Liver Physiol. 2010 Nov;299(5):G1164-76. doi: 10.1152/ajpgi.00479.2009. Epub 2010 Sep 9. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 20829522 Free PMC article.
-
Invited review: focal adhesion and small heat shock proteins in the regulation of actin remodeling and contractility in smooth muscle.J Appl Physiol (1985). 2001 Aug;91(2):963-72. doi: 10.1152/jappl.2001.91.2.963. J Appl Physiol (1985). 2001. PMID: 11457815 Review.
-
The small heat shock protein, HSPB6, in muscle function and disease.Cell Stress Chaperones. 2010 Jan;15(1):1-11. doi: 10.1007/s12192-009-0127-8. Epub 2009 Jul 1. Cell Stress Chaperones. 2010. PMID: 19568960 Free PMC article. Review.
Cited by
-
Gene expression analysis in asthma using a targeted multiplex array.BMC Pulm Med. 2017 Dec 11;17(1):189. doi: 10.1186/s12890-017-0545-9. BMC Pulm Med. 2017. PMID: 29228930 Free PMC article.
-
Loss of proteostatic control as a substrate for atrial fibrillation: a novel target for upstream therapy by heat shock proteins.Front Physiol. 2012 Feb 23;3:36. doi: 10.3389/fphys.2012.00036. eCollection 2012. Front Physiol. 2012. PMID: 22375124 Free PMC article.
-
Rho-associated kinase and zipper-interacting protein kinase, but not myosin light chain kinase, are involved in the regulation of myosin phosphorylation in serum-stimulated human arterial smooth muscle cells.PLoS One. 2019 Dec 13;14(12):e0226406. doi: 10.1371/journal.pone.0226406. eCollection 2019. PLoS One. 2019. PMID: 31834925 Free PMC article.
-
Theory and Applications of the (Cardio) Genomic Fabric Approach to Post-Ischemic and Hypoxia-Induced Heart Failure.J Pers Med. 2022 Jul 29;12(8):1246. doi: 10.3390/jpm12081246. J Pers Med. 2022. PMID: 36013195 Free PMC article.
-
Active components of ginger potentiate β-agonist-induced relaxation of airway smooth muscle by modulating cytoskeletal regulatory proteins.Am J Respir Cell Mol Biol. 2014 Jan;50(1):115-24. doi: 10.1165/rcmb.2013-0133OC. Am J Respir Cell Mol Biol. 2014. PMID: 23962082 Free PMC article.
References
-
- An SS, Fabry B, Mellema M, Bursac P, Gerthoffer WT, Kayyali US, et al. Role of heat shock protein 27 in cytoskeletal remodeling of the airway smooth muscle cell. J Appl Physiol. 2004;96:1701–1713. - PubMed
-
- Anckar J, Sistonen L. Heat shock factor 1 as a coordinator of stress and developmental pathways. Adv Exp Med Biol. 2007;594:78–88. - PubMed
-
- Arrigo AP. The cellular "networking" of mammalian Hsp27 and its functions in the control of protein folding, redox state and apoptosis. Adv Exp Med Biol. 2007;594:14–26. - PubMed
-
- Arrigo AP, Landry J. Expression and function of the low-molecular-weight heat shock proteins. In: Morimoto RI, Tissieres A, Georgopoulos C, editors. The Biology of Heat Shock Proteins and Molecular Chaperons. New York: Cold Spring Harbor Laboratory Press; 1994. pp. 335–374.
-
- Batts TW, Klausner AP, Jin Z, Meeks MK, Ripley ML, Yang SK, et al. Increased expression of heat shock protein 20 and decreased contractile stress in obstructed rat bladder. J Urol. 2006;176:1679–1684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

