Association of respiratory syncytial virus M protein with viral nucleocapsids is mediated by the M2-1 protein
- PMID: 18579594
- PMCID: PMC2519653
- DOI: 10.1128/JVI.00343-08
Association of respiratory syncytial virus M protein with viral nucleocapsids is mediated by the M2-1 protein
Abstract
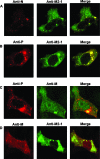
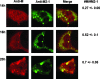
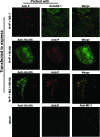
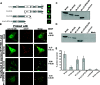
Cytoplasmic inclusions in respiratory syncytial virus-infected cells comprising viral nucleocapsid proteins (L, N, P, and M2-1) and the viral genome are sites of viral transcription. Although not believed to be necessary for transcription, the matrix (M) protein is also present in these inclusions, and we have previously shown that M inhibits viral transcription. In this study, we have investigated the mechanisms for the association of the M protein with cytoplasmic inclusions. Our data demonstrate for the first time that the M protein associates with cytoplasmic inclusions via an interaction with the M2-1 protein. The M protein colocalizes with M2-1 in the cytoplasm of cells expressing only the M and M2-1 proteins and directly interacts with M2-1 in a cell-free binding assay. Using a cotransfection system, we confirmed that the N and P proteins are sufficient to form cytoplasmic inclusions and that M2-1 localizes to these inclusions; additionally, we show that M associates with cytoplasmic inclusions only in the presence of the M2-1 protein. Using truncated mutants, we show that the N-terminal 110 amino acids of M mediate the interaction with M2-1 and the subsequent association with nucleocapsids. The interaction of M2-1 with M and, in particular, the N-terminal region of M may represent a target for novel antivirals that block the association of M with nucleocapsids, thereby inhibiting virus assembly.
Figures

References
-
- Carromeu, C., F. M. Simabuco, R. E. Tamura, L. E. F. Arcieri, and A. M. Ventura. 2007. Intracellular localization of human respiratory syncytial virus L protein. Arch. Virol. 1522259-2263. - PubMed
-
- Collins, P. L., R. M. Chanock, and B. R. Murphy. 2001. Respiratory syncytial viruses, p. 1443-1485. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, PA.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

