Protein kinase A-dependent step(s) in hepatitis C virus entry and infectivity
- PMID: 18579596
- PMCID: PMC2519651
- DOI: 10.1128/JVI.00592-08
Protein kinase A-dependent step(s) in hepatitis C virus entry and infectivity
Abstract
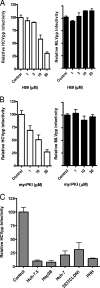
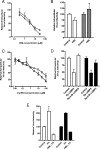
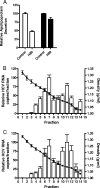
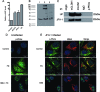
Viruses exploit signaling pathways to their advantage during multiple stages of their life cycle. We demonstrate a role for protein kinase A (PKA) in the hepatitis C virus (HCV) life cycle. The inhibition of PKA with H89, cyclic AMP (cAMP) antagonists, or the protein kinase inhibitor peptide reduced HCV entry into Huh-7.5 hepatoma cells. Bioluminescence resonance energy transfer methodology allowed us to investigate the PKA isoform specificity of the cAMP antagonists in Huh-7.5 cells, suggesting a role for PKA type II in HCV internalization. Since viral entry is dependent on the host cell expression of CD81, scavenger receptor BI, and claudin-1 (CLDN1), we studied the role of PKA in regulating viral receptor localization by confocal imaging and fluorescence resonance energy transfer (FRET) analysis. Inhibiting PKA activity in Huh-7.5 cells induced a reorganization of CLDN1 from the plasma membrane to an intracellular vesicular location(s) and disrupted FRET between CLDN1 and CD81, demonstrating the importance of CLDN1 expression at the plasma membrane for viral receptor activity. Inhibiting PKA activity in Huh-7.5 cells reduced the infectivity of extracellular virus without modulating the level of cell-free HCV RNA, suggesting that particle secretion was not affected but that specific infectivity was reduced. Viral particles released from H89-treated cells displayed the same range of buoyant densities as did those from control cells, suggesting that viral protein association with lipoproteins is not regulated by PKA. HCV infection of Huh-7.5 cells increased cAMP levels and phosphorylated PKA substrates, supporting a model where infection activates PKA in a cAMP-dependent manner to promote virus release and transmission.
Figures

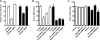
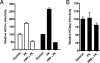

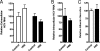
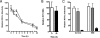
References
-
- Alto, N. M., S. H. Soderling, N. Hoshi, L. K. Langeberg, R. Fayos, P. A. Jennings, and J. D. Scott. 2003. Bioinformatic design of A-kinase anchoring protein-in silico: a potent and selective peptide antagonist of type II protein kinase A anchoring. Proc. Natl. Acad. Sci. USA 1004445-4450. - PMC - PubMed
-
- Aoubala, M., J. Holt, R. A. Clegg, D. J. Rowlands, and M. Harris. 2001. The inhibition of cAMP-dependent protein kinase by full-length hepatitis C virus NS3/4A complex is due to ATP hydrolysis. J. Gen. Virol. 821637-1646. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

