Linker histones are mobilized during infection with herpes simplex virus type 1
- PMID: 18579611
- PMCID: PMC2519646
- DOI: 10.1128/JVI.00616-08
Linker histones are mobilized during infection with herpes simplex virus type 1
Abstract
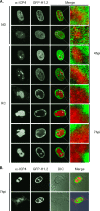
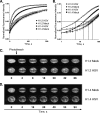
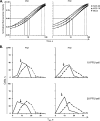
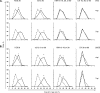
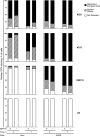
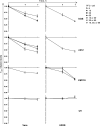
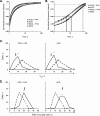
Histones interact with herpes simplex virus type 1 (HSV-1) genomes and localize to replication compartments early during infections. However, HSV-1 genomes do not interact with histones in virions and are deposited in nuclear domains devoid of histones. Moreover, late viral replication compartments are also devoid of histones. The processes whereby histones come to interact with HSV-1 genomes, to be later displaced, remain unknown. However, they would involve the early movement of histones to the domains containing HSV-1 genomes and the later movement away from them. Histones unbind from chromatin, diffuse through the nucleoplasm, and rebind at different sites. Such mobility is upregulated by, for example, phosphorylation or acetylation. We evaluated whether HSV-1 infection modulates histone mobility, using fluorescence recovery after photobleaching. All somatic H1 variants were mobilized to different degrees. H1.2, the most mobilized, was mobilized at 4 h and further so at 7 h after infection, resulting in increases in its "free" pools. H1.2 was mobilized to a "basal" degree under conditions of little to no HSV-1 protein expression. This basal mobilization required nuclear native HSV-1 genomes but was independent of HSV-1 proteins and most likely due to cellular responses. Mobilization above this basal degree, and increases in H1.2 free pools, however, depended on immediate-early or early HSV-1 proteins, but not on HSV-1 genome replication or late proteins. Linker histone mobilization is a novel consequence of cell-virus interactions, which is consistent with the dynamic interactions between histones and HSV-1 genomes during lytic infection; it may also participate in the regulation of viral gene expression.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

