Effects of multispecies probiotic combination on helicobacter pylori infection in vitro
- PMID: 18579692
- PMCID: PMC2546684
- DOI: 10.1128/CVI.00080-08
Effects of multispecies probiotic combination on helicobacter pylori infection in vitro
Abstract
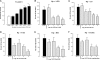
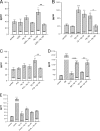
Probiotic bacteria alleviate many gastrointestinal symptoms, but the current trend of combining bacteria for additional benefit may make their effects more complex. We characterize four probiotics and their combination in terms of pathogen adhesion, barrier function, cell death, and inflammatory response in Helicobacter pylori-infected epithelial cells. H. pylori-infected Caco-2 cells were pretreated with Lactobacillus rhamnosus GG, Lactobacillus rhamnosus Lc705, Propionibacterium freudenreichii subsp. shermanii Js, Bifidobacterium breve Bb99, or all four organisms in combination. We evaluated the adhesion of H. pylori by in situ immunofluorescence; epithelial barrier function by measurement of transepithelial resistance; apoptosis by measurement of caspase 3 activation; cell membrane leakage by measurement of lactate dehydrogenase release; and inflammation by measurement of interleukin-8 (IL-8), IL-10, prostaglandin E(2) (PGE(2)), and leukotriene B(4) (LTB(4)) release. All probiotics inhibited H. pylori adhesion. L. rhamnosus GG, L. rhamnosus Lc705, P. freudenreichii subsp. shermanii Js, and the combination inhibited H. pylori-induced cell membrane leakage. L. rhamnosus GG, L. rhamnosus Lc705, and the combination initially improved epithelial barrier function but increased the H. pylori-induced barrier deterioration after incubation for 24 to 42 h. L. rhamnosus GG, L. rhamnosus Lc705, and P. freudenreichii subsp. shermanii Js inhibited H. pylori-induced IL-8 release, whereas L. rhamnosus GG, L. rhamnosus Lc705, and B. breve Bb99 suppressed PGE(2) release. None of these anti-inflammatory effects persisted when the probiotics were used in combination. The combination thus increased the levels of IL-8, PGE(2), and LTB(4) released from H. pylori-infected epithelial cells. The proinflammatory actions of the individual components dominated the anti-inflammatory effects when the probiotic bacteria were used in combination. Our results stress that the therapeutic response can be optimized if probiotic strains are characterized before they are used in combination.
Figures

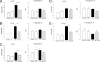

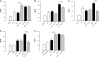

Similar articles
-
Development of new probiotics by strain combinations: is it possible to improve the adhesion to intestinal mucus?J Dairy Sci. 2007 Jun;90(6):2710-6. doi: 10.3168/jds.2006-456. J Dairy Sci. 2007. PMID: 17517710
-
Effects of anti-Helicobacter pylori treatment and probiotic supplementation on intestinal microbiota.Int J Antimicrob Agents. 2007 Jan;29(1):66-72. doi: 10.1016/j.ijantimicag.2006.08.034. Epub 2006 Dec 1. Int J Antimicrob Agents. 2007. PMID: 17141481 Clinical Trial.
-
Protection mechanism of probiotic combination against human pathogens: in vitro adhesion to human intestinal mucus.Asia Pac J Clin Nutr. 2006;15(4):570-5. Asia Pac J Clin Nutr. 2006. PMID: 17077078 Review.
-
Clinical studies on alleviating the symptoms of irritable bowel syndrome.Asia Pac J Clin Nutr. 2006;15(4):576-80. Asia Pac J Clin Nutr. 2006. PMID: 17077079 Clinical Trial.
-
The role of probiotics in the treatment and prevention of Helicobacter pylori infection.Int J Antimicrob Agents. 2003 Oct;22(4):360-6. doi: 10.1016/s0924-8579(03)00153-5. Int J Antimicrob Agents. 2003. PMID: 14522098 Review.
Cited by
-
Saccharomyces boulardii enhances anti-inflammatory effectors and AhR activation via metabolic interactions in probiotic communities.ISME J. 2024 Jan 8;18(1):wrae212. doi: 10.1093/ismejo/wrae212. ISME J. 2024. PMID: 39488793 Free PMC article.
-
Invitro synergistic activity of lactic acid bacteria against multi-drug resistant staphylococci.BMC Complement Altern Med. 2019 Mar 19;19(1):70. doi: 10.1186/s12906-019-2470-3. BMC Complement Altern Med. 2019. PMID: 30890126 Free PMC article.
-
Lyophilized cell-free supernatants of Limosilactobacillus fermentum T0701 exhibited antibacterial activity against Helicobacter pylori.Sci Rep. 2024 Jun 13;14(1):13632. doi: 10.1038/s41598-024-64443-4. Sci Rep. 2024. PMID: 38871850 Free PMC article.
-
Evaluation of the potential inhibitory activity of a combination of L. acidophilus, L. rhamnosus and L. sporogenes on Helicobacter pylori: A randomized double-blind placebo-controlled clinical trial.Chin J Integr Med. 2017 Mar;23(3):176-182. doi: 10.1007/s11655-016-2531-0. Epub 2016 Oct 19. Chin J Integr Med. 2017. PMID: 27761791 Clinical Trial.
-
Functional cell models of the gut and their applications in food microbiology--a review.Int J Food Microbiol. 2010 Jul 31;141 Suppl 1:S4-14. doi: 10.1016/j.ijfoodmicro.2010.03.026. Epub 2010 Apr 2. Int J Food Microbiol. 2010. PMID: 20444515 Free PMC article. Review.
References
-
- Bizik, J., E. Kankuri, A. Ristimäki, A. Taieb, H. Vapaatalo, W. Lubitz, and A. Vaheri. 2004. Cell-cell contacts trigger programmed necrosis and induce cyclooxygenase-2 expression. Cell Death Differ. 11:183-195. - PubMed
-
- Blum, S., Y. Delneste, S. Alvarez, D. Haller, P. F. Perez, C. Bode, W. P. Hammes, A. M. A. Pfeifer, and E. J. Schiffrin. 1999. Interactions between commensal bacteria and mucosal immunocompetent cells. Int. Dairy J. 9:63-68.
-
- Bodger, K., and J. E. Crabtree. 1998. Helicobacter pylori and gastric inflammation. Br. Med. Bull. 54:139-150. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

