NMDA receptor blockade with memantine attenuates white matter injury in a rat model of periventricular leukomalacia
- PMID: 18579741
- PMCID: PMC2800040
- DOI: 10.1523/JNEUROSCI.1702-08.2008
NMDA receptor blockade with memantine attenuates white matter injury in a rat model of periventricular leukomalacia
Abstract
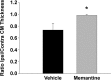
Hypoxia-ischemia (H/I) in the premature infant leads to white matter injury termed periventricular leukomalacia (PVL), the leading cause of subsequent neurological deficits. Glutamatergic excitotoxicity in white matter oligodendrocytes (OLs) mediated by cell surface glutamate receptors (GluRs) of the AMPA subtype has been demonstrated as one factor in this injury. Recently, it has been shown that rodent OLs also express functional NMDA GluRs (NMDARs), and overactivation of these receptors can mediate excitotoxic OL injury. Here we show that preterm human developing OLs express NMDARs during the PVL period of susceptibility, presenting a potential therapeutic target. The expression pattern mirrors that seen in the immature rat. Furthermore, the uncompetitive NMDAR antagonist memantine attenuates NMDA-evoked currents in developing OLs in situ in cerebral white matter of immature rats. Using an H/I rat model of white matter injury, we show in vivo that post-H/I treatment with memantine attenuates acute loss of the developing OL cell surface marker O1 and the mature OL marker MBP (myelin basic protein), and also prevents the long-term reduction in cerebral mantle thickness seen at postnatal day 21 in this model. These protective doses of memantine do not affect normal myelination or cortical growth. Together, these data suggest that NMDAR blockade with memantine may provide an effective pharmacological prevention of PVL in the premature infant.
Figures





References
-
- Block F, Schwarz M. Memantine reduces functional and morphological consequences induced by global ischemia in rats. Neurosci Lett. 1996;208:41–44. - PubMed
-
- Cai Z, Lin S, Fan LW, Pang Y, Rhodes PG. Minocycline alleviates hypoxic-ischemic injury to developing oligodendrocytes in the neonatal rat brain. Neuroscience. 2006;137:425–435. - PubMed
-
- Chen HS, Lipton SA. The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem. 2006;97:1611–1626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
