Theta and gamma coordination of hippocampal networks during waking and rapid eye movement sleep
- PMID: 18579747
- PMCID: PMC2596978
- DOI: 10.1523/JNEUROSCI.1227-08.2008
Theta and gamma coordination of hippocampal networks during waking and rapid eye movement sleep
Abstract
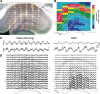
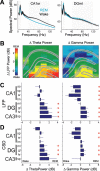
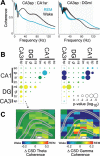
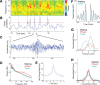
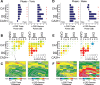
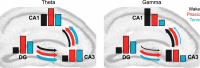
Rapid eye movement (REM) sleep has been considered a paradoxical state because, despite the high behavioral threshold to arousing perturbations, gross physiological patterns in the forebrain resemble those of waking states. To understand how intrahippocampal networks interact during REM sleep, we used 96 site silicon probes to record from different hippocampal subregions and compared the patterns of activity during waking exploration and REM sleep. Dentate/CA3 theta and gamma synchrony was significantly higher during REM sleep compared with active waking. In contrast, gamma power in CA1 and CA3-CA1 gamma coherence showed significant decreases in REM sleep. Changes in unit firing rhythmicity and unit-field coherence specified the local generation of these patterns. Although these patterns of hippocampal network coordination characterized the more common tonic periods of REM sleep (approximately 95% of total REM), we also detected large phasic bursts of local field potential power in the dentate molecular layer that were accompanied by transient increases in the firing of dentate and CA1 neurons. In contrast to tonic REM periods, phasic REM epochs were characterized by higher theta and gamma synchrony among the dentate, CA3, and CA1 regions. These data suggest enhanced dentate processing, but limited CA3-CA1 coordination during tonic REM sleep. In contrast, phasic bursts of activity during REM sleep may provide windows of opportunity to synchronize the hippocampal trisynaptic loop and increase output to cortical targets. We hypothesize that tonic REM sleep may support off-line mnemonic processing, whereas phasic bursts of activity during REM may promote memory consolidation.
Figures

References
-
- Amaral DG, Witter MP. Hippocampal formation. In: Paxinos G, editor. The rat nervous system. New York: Academic; 1995. pp. 443–490.
-
- Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953;118:273–274. - PubMed
-
- Bartho P, Hirase H, Monconduit L, Zugaro M, Harris KD, Buzsaki G. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J Neurophysiol. 2004;92:600–608. - PubMed
-
- Bastuji H, Garcia-Larrea L. Evoked potentials as a tool for the investigation of human sleep. Sleep Med Rev. 1999;3:23–45. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
