Calcium control of endocytic capacity at a CNS synapse
- PMID: 18579748
- PMCID: PMC2671224
- DOI: 10.1523/JNEUROSCI.1082-08.2008
Calcium control of endocytic capacity at a CNS synapse
Abstract
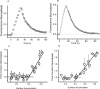
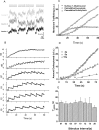
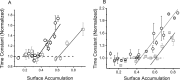
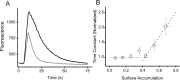
The ability to recycle synaptic vesicles is a crucial property of nerve terminals that allows maintenance of synaptic transmission. Using high-sensitivity optical approaches at hippocampal nerve terminals in dissociated neurons in culture, we show that modulation of endocytosis can be achieved by expansion of the endocytic capacity. Our experiments indicate that the endocytic capacity, the maximum number of synaptic vesicles that can be internalized in parallel at individual synapses, is tightly controlled by intracellular calcium levels. Increasing levels of intracellular calcium, which occurs as firing frequency increases, significantly increases the endocytic capacity. At physiological temperature after 30 Hz firing, these synapses are capable of endocytosing at least approximately 28 vesicles in parallel, each with a time constant of approximately 6 s. This calcium-dependent control of endocytic capacity reveals a potentially useful adaptive response to high-frequency activity to increase endocytic rates under conditions of vesicle pool depletion.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
