Selective degeneration of central photoreceptors after hyperbaric oxygen in normal and metallothionein-knockout mice
- PMID: 18579766
- PMCID: PMC2709831
- DOI: 10.1167/iovs.07-1039
Selective degeneration of central photoreceptors after hyperbaric oxygen in normal and metallothionein-knockout mice
Abstract
Purpose: Metallothioneins (MTs) in the brain and retina are believed to bind metals and reduce free radicals, thereby protecting neurons from oxidative damage. This study was undertaken to investigate whether retinal photoreceptor (PR) cells lacking MTs are more susceptible to hyperbaric oxygen (HBO)-induced cell death in vivo.
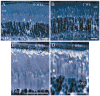
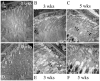
Methods: Wild-type (WT) and MT-knockout (MT-KO) mice lacking metallothionein (MT)-1 and MT-2 were exposed to three atmospheres of 100% oxygen for 3 hours, 3 times per week for 1, 3, or 5 weeks. The control animals were not exposed. Histologic analysis of PR viability was performed by counting rows of nuclei in the outer nuclear layer (ONL). Ultrastructure studies verified PR damage.
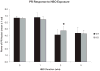
Results: HBO exposure produced a major loss of PR cells in the central retinas of WT and MT-KO mice, with no effect on the peripheral retina even at the longest (5 weeks) exposures. The degree of PR damage and cell death increased with duration of HBO exposure. One week of HBO exposure was insufficient to cause PR death, but tissue damage was observed in the inner and outer segments. At 3 weeks, the rows of PR nuclei in the central retina were significantly reduced by 38% in WT and 28% in MT-KO animals. At 5 weeks, PR loss was identical in WT (34%) and MT-KO (34%) animals and was comparable to that in WT at 3 weeks.
Conclusions: The data suggest that MT-1 and -2 alone are not sufficient for protecting PRs against HBO-induced cell death. The selective degeneration of central PRs may provide clues to mechanisms of oxidative damage in retinal disease.
Figures




Similar articles
-
Metallothionein-III deficiency exacerbates light-induced retinal degeneration.Invest Ophthalmol Vis Sci. 2012 Nov 29;53(12):7896-903. doi: 10.1167/iovs.12-10165. Invest Ophthalmol Vis Sci. 2012. PMID: 23132798
-
Loss of protein kinase Cgamma in knockout mice and increased retinal sensitivity to hyperbaric oxygen.Arch Ophthalmol. 2009 Apr;127(4):500-6. doi: 10.1001/archophthalmol.2009.31. Arch Ophthalmol. 2009. PMID: 19365031 Free PMC article.
-
Role of heparin-binding epidermal growth factor-like growth factor in light-induced photoreceptor degeneration in mouse retina.Invest Ophthalmol Vis Sci. 2013 Jun 3;54(6):3815-29. doi: 10.1167/iovs.12-11236. Invest Ophthalmol Vis Sci. 2013. PMID: 23640042
-
Suppression of developmental retinal cell death but not of photoreceptor degeneration in Bax-deficient mice.Invest Ophthalmol Vis Sci. 1998 Aug;39(9):1713-20. Invest Ophthalmol Vis Sci. 1998. PMID: 9699561
-
Estrogen, via ESR2 receptor, prevents oxidative stress-induced Müller cell death and stimulates FGF2 production independently of NRF2, attenuating retinal degeneration.Exp Eye Res. 2024 Nov;248:110103. doi: 10.1016/j.exer.2024.110103. Epub 2024 Sep 18. Exp Eye Res. 2024. PMID: 39303841
Cited by
-
Metallothionein-induced zinc partitioning exacerbates hyperoxic acute lung injury.Am J Physiol Lung Cell Mol Physiol. 2013 Mar 1;304(5):L350-60. doi: 10.1152/ajplung.00243.2012. Epub 2012 Dec 28. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23275622 Free PMC article.
-
Prognostic factors of hyperbaric oxygen therapy for patients with delayed encephalopathy after acute carbon monoxide poisoning.Heliyon. 2022 Dec 15;8(12):e12351. doi: 10.1016/j.heliyon.2022.e12351. eCollection 2022 Dec. Heliyon. 2022. PMID: 36582705 Free PMC article.
-
Dietary supplementation of blueberry juice enhances hepatic expression of metallothionein and attenuates liver fibrosis in rats.PLoS One. 2013;8(3):e58659. doi: 10.1371/journal.pone.0058659. Epub 2013 Mar 12. PLoS One. 2013. PMID: 23554912 Free PMC article.
-
Immunogenetic and Environmental Factors in Age-Related Macular Disease.Int J Mol Sci. 2024 Jun 14;25(12):6567. doi: 10.3390/ijms25126567. Int J Mol Sci. 2024. PMID: 38928273 Free PMC article. Review.
-
Differential gene expression in mouse retina related to regional differences in vulnerability to hyperoxia.Mol Vis. 2010 Apr 28;16:740-55. Mol Vis. 2010. PMID: 20454693 Free PMC article.
References
-
- Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med. 2004;10(suppl):S18–S25. - PubMed
-
- Miles AT, Hawksworth GM, Beattie JH, Rodilla V. Induction, regulation, degradation, and biological significance of mammalian metallothioneins. Crit Rev Biochem Mol Biol. 2000;35:35–70. - PubMed
-
- Cai L, Koropatnick J, Cherian MG. Metallothionein protects DNA from copper-induced but not iron-induced cleavage in vitro. Chem Biol Interact. 1995;96:143–155. - PubMed
-
- Kang YJ. The antioxidant function of metallothionein in the heart. Proc Soc Exp Biol Med. 1999;222:263–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

