An epoxide hydrolase involved in the biosynthesis of an insect sex attractant and its use to localize the production site
- PMID: 18579785
- PMCID: PMC2449339
- DOI: 10.1073/pnas.0801559105
An epoxide hydrolase involved in the biosynthesis of an insect sex attractant and its use to localize the production site
Abstract
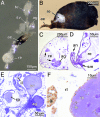
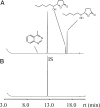
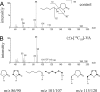
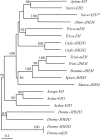
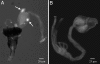
Epoxide hydrolases (EHs) are enzymes occurring in virtually any living organism. They catalyze the hydrolysis of epoxide containing lipids and are involved in crucial mechanisms, such as the detoxification of xenobiotics or the regulation of inflammation and blood pressure. Here, we describe a function of a putative EH gene in the biosynthesis of a sex attractant in the jewel wasp Nasonia vitripennis and use this gene to localize the site of pheromone production. Males of this parasitic wasp release a mixture of (4R,5R)-( threo-) and (4R,5S)-( erythro-)5-hydroxy-4-decanolide (HDL) to attract virgin females. Using a stable isotope labeled precursor, we demonstrated that vernolic acid ( erythro-12,13-epoxy-octadec-9Z-enoic acid) is converted by N. vitripennis males to threo-HDL. This suggested the involvement of an EH in hydrolyzing the fatty acid epoxide under inversion of the stereochemistry into the respective diol, which might be further processed by chain shortening and lactonization to HDL. We cloned a putative N. vitripennis EH gene (Nasvi-EH1) encoding 470 amino acids and localized its transcripts in the male rectal papillae by in situ RT-PCR. Chemical analyses and histological studies confirmed that males synthesize the sex attractant in the rectal vesicle and release it via the anal orifice. Involvement of Nasvi-EH1 in HDL biosynthesis was established by RNAi-mediated gene silencing. Injection of Nasvi-EH1 dsRNA into male abdomens inhibited pheromone biosynthesis by 55% and suppressed the targeted gene transcripts in the rectal vesicle by 95%.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
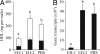
References
-
- Hardie J, Minks AK. Pheromones of Non-Lepidopteran Insects Associated with Agricultural Plants. Wallingford, CT: CABI; 1999.
-
- Wyatt TD. Pheromones and Animal Behaviour. Cambridge: Cambridge Univ Press; 2003.
-
- Ma PWK, Ramaswamy SB. Biology and ultrastructure of sex pheromone-producing tissue. In: Blomquist G, Vogt R, editors. Insect Pheromone Biochemistry and Molecular Biology. London: Elsevier; 2003. pp. 19–51.
-
- Tillman JA, Seybold SJ, Jurenka RA, Blomquist GJ. Insect pheromones—an overview of biosynthesis and endocrine regulation. Insect Biochem Mol Biol. 1999;29:481–514. - PubMed
-
- Jurenka RA. Insect pheromone biosynthesis. Top Curr Chem. 2004;239:97–132. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

