Oncogenic Kit controls neoplastic mast cell growth through a Stat5/PI3-kinase signaling cascade
- PMID: 18579792
- PMCID: PMC2532813
- DOI: 10.1182/blood-2007-09-115477
Oncogenic Kit controls neoplastic mast cell growth through a Stat5/PI3-kinase signaling cascade
Erratum in
- Blood. 2009 Aug 20;114(8):1720
Abstract
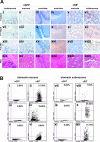
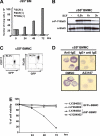
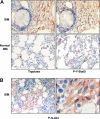
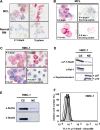
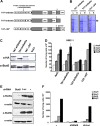
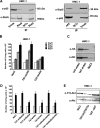
The D816V-mutated variant of Kit triggers multiple signaling pathways and is considered essential for malignant transformation in mast cell (MC) neoplasms. We here describe that constitutive activation of the Stat5-PI3K-Akt-cascade controls neoplastic MC development. Retrovirally transduced active Stat5 (cS5(F)) was found to trigger PI3K and Akt activation, and to transform murine bone marrow progenitors into tissue-infiltrating MCs. Primary neoplastic Kit D816V(+) MCs in patients with mastocytosis also displayed activated Stat5, which was found to localize to the cytoplasm and to form a signaling complex with PI3K, with consecutive Akt activation. Finally, the knock-down of either Stat5 or Akt activity resulted in growth inhibition of neoplastic Kit D816V(+) MCs. These data suggest that a downstream Stat5-PI3K-Akt signaling cascade is essential for Kit D816V-mediated growth and survival of neoplastic MCs.
Figures

References
-
- Austen KF, Boyce JA. Mast cell lineage development and phenotypic regulation. Leuk Res. 2001;25:511–518. - PubMed
-
- Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. - PubMed
-
- Valent P, Horny HP, Escribano L, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25:603–625. - PubMed
-
- Valent P, Akin C, Sperr WR, et al. Diagnosis and treatment of systemic mastocytosis: state of the art. Br J Haematol. 2003;122:695–717. - PubMed
-
- Akin C, Metcalfe DD. Systemic mastocytosis. Annu Rev Med. 2004;55:419–432. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

