Rac GTPase isoforms Rac1 and Rac2 play a redundant and crucial role in T-cell development
- PMID: 18579797
- PMCID: PMC2518885
- DOI: 10.1182/blood-2008-01-132068
Rac GTPase isoforms Rac1 and Rac2 play a redundant and crucial role in T-cell development
Abstract
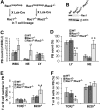
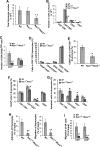
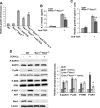
Rac GTPases have been implicated in the regulation of diverse functions in various blood cell lineages, but their role in T-cell development is not well understood. We have carried out conditional gene targeting to achieve hematopoietic stem cell (HSC)- or T-cell lineage-specific deletion of Rac1 or Rac1/Rac2 by crossbreeding the Mx-Cre or Lck-Cre transgenic mice with Rac1(loxp/loxp) or Rac1(loxp/loxp);Rac2(-/-) mice. We found that (1) HSC deletion of both Rac1 and Rac2 inhibited production of common lymphoid progenitors (CLPs) in bone marrow and suppressed T-cell development in thymus and peripheral organs, whereas deletion of Rac1 moderately affected CLP production and T-cell development. (2) T cell-specific deletion of Rac1 did not affect T-cell development, whereas deletion of both Rac1 and Rac2 reduced immature CD4(+)CD8(+) and mature CD4(+) populations in thymus as well as CD4(+) and CD8(+) populations in spleen. (3) The developmental defects of Rac1/Rac2 knockout T cells were associated with proliferation, survival, adhesion, and migration defects. (4) Rac1/Rac2 deletion suppressed T-cell receptor-mediated proliferation, IL-2 production, and Akt activation in thymocytes. Thus, Rac1 and Rac2 have unique roles in CLP production and share a redundant but essential role in later stages of T-cell development by regulating survival and proliferation signals.
Figures


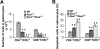

Similar articles
-
Rac GTPases play critical roles in early T-cell development.Blood. 2009 Apr 23;113(17):3990-8. doi: 10.1182/blood-2008-09-181180. Epub 2008 Dec 16. Blood. 2009. PMID: 19088377 Free PMC article.
-
Rac1 and Rac2 GTPases are necessary for early erythropoietic expansion in the bone marrow but not in the spleen.Haematologica. 2010 Jan;95(1):27-35. doi: 10.3324/haematol.2009.006239. Haematologica. 2010. PMID: 20065081 Free PMC article.
-
Identifying the relative contributions of Rac1 and Rac2 to osteoclastogenesis.J Bone Miner Res. 2008 Feb;23(2):260-70. doi: 10.1359/jbmr.071013. J Bone Miner Res. 2008. PMID: 17922611
-
Rho GTPases and regulation of hematopoietic stem cell localization.Methods Enzymol. 2008;439:365-93. doi: 10.1016/S0076-6879(07)00427-2. Methods Enzymol. 2008. PMID: 18374178 Review.
-
Knocked out by Rho/Rac T-cell biology.Histol Histopathol. 2002;17(3):871-5. doi: 10.14670/HH-17.871. Histol Histopathol. 2002. PMID: 12168798 Review.
Cited by
-
Rac1/2 activation promotes FGFR1 driven leukemogenesis in stem cell leukemia/lymphoma syndrome.Haematologica. 2020 Jan 31;105(2):e68-e71. doi: 10.3324/haematol.2018.208058. Print 2020. Haematologica. 2020. PMID: 31221776 Free PMC article. No abstract available.
-
Thiopurine Prodrugs Mediate Immunosuppressive Effects by Interfering with Rac1 Protein Function.J Biol Chem. 2016 Jun 24;291(26):13699-714. doi: 10.1074/jbc.M115.694422. Epub 2016 May 9. J Biol Chem. 2016. PMID: 27189938 Free PMC article.
-
Distinct roles of Cdc42 in thymopoiesis and effector and memory T cell differentiation.PLoS One. 2011 Mar 24;6(3):e18002. doi: 10.1371/journal.pone.0018002. PLoS One. 2011. PMID: 21455314 Free PMC article.
-
Hematopoietic-specific Rho GTPases Rac2 and RhoH and human blood disorders.Exp Cell Res. 2013 Sep 10;319(15):2375-83. doi: 10.1016/j.yexcr.2013.07.002. Epub 2013 Jul 11. Exp Cell Res. 2013. PMID: 23850828 Free PMC article. Review.
-
Rac1 targeting suppresses p53 deficiency-mediated lymphomagenesis.Blood. 2010 Apr 22;115(16):3320-8. doi: 10.1182/blood-2009-02-202440. Epub 2010 Feb 23. Blood. 2010. PMID: 20179179 Free PMC article.
References
-
- Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. - PubMed
-
- Zheng Y. Dbl family guanine nucleotide exchange factors. Trends Biochem Sci. 2001;26:724–732. - PubMed
-
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. - PubMed
-
- Wang L, Zheng Y. Cell type specific function of Rho GTPases revealed by gene targeting in mice. Trends Cell Biol. 2007;17:58–64. - PubMed
-
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

