Copper is taken up efficiently from albumin and alpha2-macroglobulin by cultured human cells by more than one mechanism
- PMID: 18579803
- PMCID: PMC2544443
- DOI: 10.1152/ajpcell.00029.2008
Copper is taken up efficiently from albumin and alpha2-macroglobulin by cultured human cells by more than one mechanism
Abstract
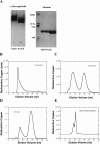
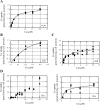
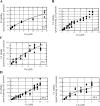
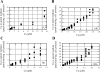
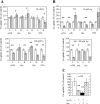
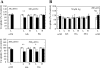
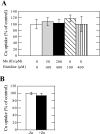
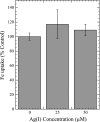
Ionic copper entering blood plasma binds tightly to albumin and the macroglobulin transcuprein. It then goes primarily to the liver and kidney except in lactation, where a large portion goes directly to the mammary gland. Little is known about how this copper is taken up from these plasma proteins. To examine this, the kinetics of uptake from purified human albumin and alpha(2)-macroglobulin, and the effects of inhibitors, were measured using human hepatic (HepG2) and mammary epithelial (PMC42) cell lines. At physiological concentrations (3-6 muM), both cell types took up copper from these proteins independently and at rates similar to each other and to those for Cu-dihistidine or Cu-nitrilotriacetate (NTA). Uptakes from alpha(2)-macroglobulin indicated a single saturable system in each cell type, but with different kinetics, and 65-80% inhibition by Ag(I) in HepG2 cells but not PMC42 cells. Uptake kinetics for Cu-albumin were more complex and also differed with cell type (as was the case for Cu-histidine and NTA), and there was little or no inhibition by Ag(I). High Fe(II) concentrations (100-500 microM) inhibited copper uptake from albumin by 20-30% in both cell types and that from alpha(2)-macroglobulin by 0-30%, and there was no inhibition of the latter by Mn(II) or Zn(II). We conclude that the proteins mainly responsible for the plasma-exchangeable copper pool deliver the metal to mammalian cells efficiently and by several different mechanisms. alpha(2)-Macroglobulin delivers it primarily to copper transporter 1 in hepatic cells but not mammary epithelial cells, and additional as-yet-unidentified copper transporters or systems for uptake from these proteins remain to be identified.
Figures

Similar articles
-
Uptake of copper from plasma proteins in cells where expression of CTR1 has been modulated.Biometals. 2012 Aug;25(4):697-709. doi: 10.1007/s10534-012-9528-8. Epub 2012 Feb 22. Biometals. 2012. PMID: 22354499
-
Transcuprein is a macroglobulin regulated by copper and iron availability.J Nutr Biochem. 2007 Sep;18(9):597-608. doi: 10.1016/j.jnutbio.2006.11.005. Epub 2007 Mar 23. J Nutr Biochem. 2007. PMID: 17363239 Free PMC article.
-
Mechanism of Copper Uptake from Blood Plasma Ceruloplasmin by Mammalian Cells.PLoS One. 2016 Mar 2;11(3):e0149516. doi: 10.1371/journal.pone.0149516. eCollection 2016. PLoS One. 2016. PMID: 26934375 Free PMC article.
-
Mechanism of copper transport from plasma to hepatocytes.Fed Proc. 1986 Nov;45(12):2800-4. Fed Proc. 1986. PMID: 3533645 Review.
-
The families of zinc (SLC30 and SLC39) and copper (SLC31) transporters.Curr Top Membr. 2014;73:321-55. doi: 10.1016/B978-0-12-800223-0.00009-8. Curr Top Membr. 2014. PMID: 24745988 Review.
Cited by
-
Engineered nanomaterials and oxidative stress: current understanding and future challenges.Curr Opin Toxicol. 2019 Feb;13:74-80. doi: 10.1016/j.cotox.2018.09.001. Epub 2018 Sep 7. Curr Opin Toxicol. 2019. PMID: 31263794 Free PMC article.
-
Does Ceruloplasmin Defend Against Neurodegenerative Diseases?Curr Neuropharmacol. 2019;17(6):539-549. doi: 10.2174/1570159X16666180508113025. Curr Neuropharmacol. 2019. PMID: 29737252 Free PMC article. Review.
-
64Cu2+ Complexes of Tripodal Amine Ligands' In Vivo Tumor and Liver Uptakes and Intracellular Cu Distribution in the Extrahepatic Bile Duct Carcinoma Cell Line TFK-1: A Basic Comparative Study.Pharmaceuticals (Basel). 2024 Jun 21;17(7):820. doi: 10.3390/ph17070820. Pharmaceuticals (Basel). 2024. PMID: 39065671 Free PMC article.
-
Size-Dependent Bioactivity of Silver Nanoparticles: Antibacterial Properties, Influence on Copper Status in Mice, and Whole-Body Turnover.Nanotechnol Sci Appl. 2020 Dec 31;13:137-157. doi: 10.2147/NSA.S287658. eCollection 2020. Nanotechnol Sci Appl. 2020. PMID: 33408467 Free PMC article.
-
Pan-cancer analyses reveal molecular and clinical characteristics of cuproptosis regulators.Imeta. 2022 Dec 7;2(1):e68. doi: 10.1002/imt2.68. eCollection 2023 Feb. Imeta. 2022. PMID: 38868340 Free PMC article.
References
-
- Ackland ML, Michalczyk A, Whitehead RH. PMC42, a novel model for the differentiated breast. Exp Cell Res 263: 14–22, 2001. - PubMed
-
- Ackland ML, Newgreen DF, Price J, Fridman M, Waltham MC, Arvanitis A, Minichiello J, Price JT, Thompson A. Epidermal growth factor-induced epithelio-mesenchymal transition in human breast carcinoma cells. Lab Invest 83: 435–448, 2003. - PubMed
-
- Arredondo M, Munoz P, Mura CV, Nunez MT. DMT1, a physiologically relevant apical Cu1+ transporter of intestinal cells. Am J Physiol Cell Physiol 284: C1525–C1530, 2003. - PubMed
-
- Barrow L, Tanner MS. Copper distribution among serum proteins in paediatric liver disorders and malignancies. Eur J Clin Invest 18: 555–560, 1988. - PubMed
-
- Bertinato J, Swist E, Plouffe LJ, Brooks SP, L'Abbé MR. Ctr2 is partially localized to the plasma membrane and stimulates copper uptake in COS-7 cells. Biochem J 409: 731–740, 2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

