Programming of human monocytes by the uteroplacental environment
- PMID: 18579853
- PMCID: PMC2673694
- DOI: 10.1177/1933719107314065
Programming of human monocytes by the uteroplacental environment
Abstract
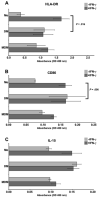
During human pregnancy, monocytes recruited to the uterus (decidua) are modified to promote immune defense and semiallogeneic pregnancy. The purpose of this study was to identify decidual factors involved in programming of monocytes into decidual macrophages by comparing the surface and secretory phenotypes of resting and interferon- gamma (IFN-gamma)-activated monocytes, unfractionated decidual cells, purified term decidual macrophages, and monocyte-derived macrophages. Surface markers for antigen presentation (HLA-DR, CD86), a membrane-bound cytokine interleukin (IL)-15, leukocyte immunoglobulin-like receptors (LILRB1, LILRB2), and secreted anti-inflammatory cytokines (transforming growth factor [TGF]-beta1 and IL-10) were assessed. The results demonstrate that differentiated, activated monocytes closely resemble but are not identical to decidual macrophages. In addition to differential IFN-gamma responsiveness, decidual macrophages were smaller than monocyte-derived macrophages and produced IL-10, which monocyte-derived macrophages did not. Only the unfractionated decidual cells secreted TGF-beta1. These results suggest that activation, differentiation, and decidual signals cooperate to program monocytes into the decidual macrophage phenotype.
Figures





Comment in
-
Macrophages and pregnancy.Reprod Sci. 2008 May;15(5):435-6. doi: 10.1177/1933719108317253. Reprod Sci. 2008. PMID: 18579852 No abstract available.
References
-
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. - PubMed
-
- Rutherford MS, Witsell A, Schook LB. Mechanisms generating functionally heterogeneous macrophages: chaos revisited. J Leukoc Biol. 1993;53:602–618. - PubMed
-
- Vince GS, Johnson PM. Immunobiology of human uteroplacental macrophages—friend and foe? Placenta. 1996;17:191–199. - PubMed
-
- Hunt JS, Pollard JW. Macrophages in the uterus and placenta. Curr Top Microbiol Immunol. 1992;181:39–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

